Krüpple-like factor 5 is required for proper maintenance of adult intestinal crypt cellular proliferation
- PMID: 25069574
- PMCID: PMC4286443
- DOI: 10.1007/s10620-014-3307-z
Krüpple-like factor 5 is required for proper maintenance of adult intestinal crypt cellular proliferation
Abstract
Background: Krüpple-like factor 5 (KLF5) is a transcription factor that is highly expressed in the proliferative compartment of the intestinal crypt. There, it is thought to regulate epithelial turnover and homeostasis.
Aim: In this study, we sought to determine the role for Klf5 in the maintenance of cellular proliferation, cytodifferentiation, and morphology of the crypt-villus axis.
Methods: Tamoxifen-induced recombination directed by the epithelial-specific Villin promoter (in Villin-CreERT2 transgenic mice) was used to delete Klf5 (in Klf5 (loxP/loxP) mice) from the adult mouse intestine and analyzed by immunostaining and RT-qPCR. Control mice were tamoxifen-treated Klf5 (loxP/loxP) mice lacking Villin-CreERT2.
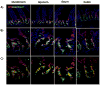
Results: Three days after tamoxifen-induced recombination, the mitosis marker phospho-histone H3 was significantly reduced within the Klf5-mutant crypt epithelium, coincident with increased expression of the apoptosis marker cleaved-caspase 3 within the crypt where cell death rarely occurs normally. We also observed a reduction in Chromagranin A expressing enteroendocrine cells, though no significant change was seen in other secretory or absorptive cell types. To examine the long-term repercussions of Klf5 loss, we killed mice 5, 14, and 28 days post recombination and found reemerging expression of KLF5. Furthermore, we observed restoration of cellular proliferation, though not to levels seen wildtype intestinal crypts. Reduction of apoptosis to levels comparable to the wildtype intestinal crypt was also observed at later time points. Analysis of cell cycle machinery indicated no significant perturbation upon deletion of Klf5; however, a reduction of stem cell markers Ascl2, Lgr5, and Olfm4 was observed at all time points following Klf5 deletion.
Conclusions: These results indicate that Klf5 is necessary to maintain adult intestinal crypt proliferation and proper cellular differentiation. Rapid replacement of Klf5-mutant crypts with wildtype cells and reduction of stem cell markers suggests further that Klf5 is required for self renewal of intestinal stem cells.
Figures












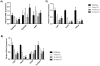
Similar articles
-
Krüppel-like factor 5 is essential for proliferation and survival of mouse intestinal epithelial stem cells.Stem Cell Res. 2015 Jan;14(1):10-9. doi: 10.1016/j.scr.2014.10.008. Epub 2014 Nov 6. Stem Cell Res. 2015. PMID: 25460247 Free PMC article.
-
Krüppel-like factor 5 is important for maintenance of crypt architecture and barrier function in mouse intestine.Gastroenterology. 2011 Oct;141(4):1302-13, 1313.e1-6. doi: 10.1053/j.gastro.2011.06.086. Epub 2011 Jul 18. Gastroenterology. 2011. PMID: 21763241 Free PMC article.
-
Krüppel-like Factor 5 Regulates Stemness, Lineage Specification, and Regeneration of Intestinal Epithelial Stem Cells.Cell Mol Gastroenterol Hepatol. 2020;9(4):587-609. doi: 10.1016/j.jcmgh.2019.11.009. Epub 2019 Nov 25. Cell Mol Gastroenterol Hepatol. 2020. PMID: 31778829 Free PMC article.
-
The diverse functions of Krüppel-like factors 4 and 5 in epithelial biology and pathobiology.Bioessays. 2007 Jun;29(6):549-57. doi: 10.1002/bies.20581. Bioessays. 2007. PMID: 17508399 Free PMC article. Review.
-
Proliferation and differentiation of the small intestinal epithelium: from Petri dish to bedside.Ital J Gastroenterol. 1994 Dec;26(9):459-70. Ital J Gastroenterol. 1994. PMID: 7599348 Review.
Cited by
-
miRNA-576 Alleviates the Malignant Progression of Atherosclerosis through Downregulating KLF5.Dis Markers. 2021 Dec 8;2021:5450685. doi: 10.1155/2021/5450685. eCollection 2021. Dis Markers. 2021. PMID: 34925646 Free PMC article.
-
The regulatory niche of intestinal stem cells.J Physiol. 2016 Sep 1;594(17):4827-36. doi: 10.1113/JP271931. Epub 2016 Jul 28. J Physiol. 2016. PMID: 27060879 Free PMC article. Review.
-
SP and KLF Transcription Factors in Digestive Physiology and Diseases.Gastroenterology. 2017 Jun;152(8):1845-1875. doi: 10.1053/j.gastro.2017.03.035. Epub 2017 Mar 30. Gastroenterology. 2017. PMID: 28366734 Free PMC article. Review.
-
The Novel Small-Molecule SR18662 Efficiently Inhibits the Growth of Colorectal Cancer In Vitro and In Vivo.Mol Cancer Ther. 2019 Nov;18(11):1973-1984. doi: 10.1158/1535-7163.MCT-18-1366. Epub 2019 Jul 29. Mol Cancer Ther. 2019. PMID: 31358661 Free PMC article.
-
Genome-Wide Analysis of Long Noncoding RNAs in Porcine Intestine during Weaning Stress.Int J Mol Sci. 2023 Mar 10;24(6):5343. doi: 10.3390/ijms24065343. Int J Mol Sci. 2023. PMID: 36982414 Free PMC article.
References
-
- Buddington RK, Diamond JM. Ontogenetic development of intestinal nutrient transporters. Annual Review of Physiology. 1989;1989(51):601–619. - PubMed
-
- Deplancke B, Gaskins HR. Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. American Journal of Clincal Nutrition. 2001;73:1131S–1141S. - PubMed
-
- Shimizu M. Modulation of intestinal functions by food substances. Die Nahrung. 1999;43(3):154–158. - PubMed
-
- Strader AD, Woods SC. Gastrointestinal hormones and food intake. Gastroenterology. 2005;128(1):175–191. - PubMed
-
- Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. The American Journal of Anatomy. 1974;141(4):537–561. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

