Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4
- PMID: 25065623
- PMCID: PMC4142105
- DOI: 10.1016/j.immuni.2014.06.014
Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4
Abstract
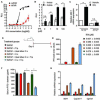
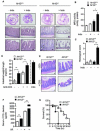
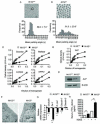
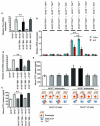
Intestinal microbial metabolites are conjectured to affect mucosal integrity through an incompletely characterized mechanism. Here we showed that microbial-specific indoles regulated intestinal barrier function through the xenobiotic sensor, pregnane X receptor (PXR). Indole 3-propionic acid (IPA), in the context of indole, is a ligand for PXR in vivo, and IPA downregulated enterocyte TNF-α while it upregulated junctional protein-coding mRNAs. PXR-deficient (Nr1i2(-/-)) mice showed a distinctly "leaky" gut physiology coupled with upregulation of the Toll-like receptor (TLR) signaling pathway. These defects in the epithelial barrier were corrected in Nr1i2(-/-)Tlr4(-/-) mice. Our results demonstrate that a direct chemical communication between the intestinal symbionts and PXR regulates mucosal integrity through a pathway that involves luminal sensing and signaling by TLR4.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Pregnane X Receptor Activation Attenuates Inflammation-Associated Intestinal Epithelial Barrier Dysfunction by Inhibiting Cytokine-Induced Myosin Light-Chain Kinase Expression and c-Jun N-Terminal Kinase 1/2 Activation.J Pharmacol Exp Ther. 2016 Oct;359(1):91-101. doi: 10.1124/jpet.116.234096. Epub 2016 Jul 20. J Pharmacol Exp Ther. 2016. PMID: 27440420 Free PMC article.
-
Lipopolysaccharide Regulation of Intestinal Tight Junction Permeability Is Mediated by TLR4 Signal Transduction Pathway Activation of FAK and MyD88.J Immunol. 2015 Nov 15;195(10):4999-5010. doi: 10.4049/jimmunol.1402598. Epub 2015 Oct 14. J Immunol. 2015. PMID: 26466961 Free PMC article.
-
Targeting the PXR-TLR4 signaling pathway to reduce intestinal inflammation in an experimental model of necrotizing enterocolitis.Pediatr Res. 2018 May;83(5):1031-1040. doi: 10.1038/pr.2018.14. Epub 2018 Feb 21. Pediatr Res. 2018. PMID: 29360809 Free PMC article.
-
Pregnane X receptor as the "sensor and effector" in regulating epigenome.J Cell Physiol. 2015 Apr;230(4):752-7. doi: 10.1002/jcp.24838. J Cell Physiol. 2015. PMID: 25294580 Review.
-
Novel functions of PXR in cardiometabolic disease.Biochim Biophys Acta. 2016 Sep;1859(9):1112-1120. doi: 10.1016/j.bbagrm.2016.02.015. Epub 2016 Feb 26. Biochim Biophys Acta. 2016. PMID: 26924429 Free PMC article. Review.
Cited by
-
Metabolic regulation of intestinal homeostasis: molecular and cellular mechanisms and diseases.MedComm (2020). 2024 Oct 25;5(11):e776. doi: 10.1002/mco2.776. eCollection 2024 Nov. MedComm (2020). 2024. PMID: 39465140 Free PMC article. Review.
-
Bacteria and Allergic Diseases.Int J Mol Sci. 2024 Sep 25;25(19):10298. doi: 10.3390/ijms251910298. Int J Mol Sci. 2024. PMID: 39408628 Free PMC article. Review.
-
Clostridium sporogenes-derived metabolites protect mice against colonic inflammation.Gut Microbes. 2024 Jan-Dec;16(1):2412669. doi: 10.1080/19490976.2024.2412669. Epub 2024 Oct 14. Gut Microbes. 2024. PMID: 39397690 Free PMC article.
-
The role of microbial indole metabolites in tumor.Gut Microbes. 2024 Jan-Dec;16(1):2409209. doi: 10.1080/19490976.2024.2409209. Epub 2024 Oct 1. Gut Microbes. 2024. PMID: 39353090 Free PMC article. Review.
-
Rifaximin ameliorates influenza A virus infection-induced lung barrier damage by regulating gut microbiota.Appl Microbiol Biotechnol. 2024 Sep 19;108(1):469. doi: 10.1007/s00253-024-13280-6. Appl Microbiol Biotechnol. 2024. PMID: 39298023 Free PMC article.
References
-
- Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. 2010;10:131–144. - PubMed
-
- Abreu MT, Vora P, Faure E, Thomas LS, Arnold ET, Arditi M. Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. Journal of immunology. 2001;167:1609–1616. - PubMed
-
- Asano T, Tanaka K, Yamakawa N, Adachi H, Sobue G, Goto H, Takeuchi K, Mizushima T. HSP70 confers protection against indomethacin-induced lesions of the small intestine. The Journal of pharmacology and experimental therapeutics. 2009;330:458–467. - PubMed
-
- Ashida H, Ogawa M, Kim M, Mimuro H, Sasakawa C. Bacteria and host interactions in the gut epithelial barrier. Nat Chem Biol. 2011;8:36–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

