The semen microbiome and its relationship with local immunology and viral load in HIV infection
- PMID: 25058515
- PMCID: PMC4110035
- DOI: 10.1371/journal.ppat.1004262
The semen microbiome and its relationship with local immunology and viral load in HIV infection
Abstract
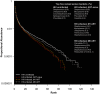
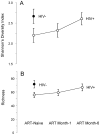
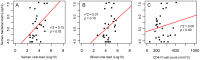
Semen is a major vector for HIV transmission, but the semen HIV RNA viral load (VL) only correlates moderately with the blood VL. Viral shedding can be enhanced by genital infections and associated inflammation, but it can also occur in the absence of classical pathogens. Thus, we hypothesized that a dysregulated semen microbiome correlates with local HIV shedding. We analyzed semen samples from 49 men who have sex with men (MSM), including 22 HIV-uninfected and 27 HIV-infected men, at baseline and after starting antiretroviral therapy (ART) using 16S rRNA gene-based pyrosequencing and quantitative PCR. We studied the relationship of semen bacteria with HIV infection, semen cytokine levels, and semen VL by linear regression, non-metric multidimensional scaling, and goodness-of-fit test. Streptococcus, Corynebacterium, and Staphylococcus were common semen bacteria, irrespective of HIV status. While Ureaplasma was the more abundant Mollicutes in HIV-uninfected men, Mycoplasma dominated after HIV infection. HIV infection was associated with decreased semen microbiome diversity and richness, which were restored after six months of ART. In HIV-infected men, semen bacterial load correlated with seven pro-inflammatory semen cytokines, including IL-6 (p = 0.024), TNF-α (p = 0.009), and IL-1b (p = 0.002). IL-1b in particular was associated with semen VL (r(2) = 0.18, p = 0.02). Semen bacterial load was also directly linked to the semen HIV VL (r(2) = 0.15, p = 0.02). HIV infection reshapes the relationship between semen bacteria and pro-inflammatory cytokines, and both are linked to semen VL, which supports a role of the semen microbiome in HIV sexual transmission.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Semen Bacterial Concentrations and HIV-1 RNA Shedding Among HIV-1-Seropositive Kenyan Men.J Acquir Immune Defic Syndr. 2017 Mar 1;74(3):250-257. doi: 10.1097/QAI.0000000000001244. J Acquir Immune Defic Syndr. 2017. PMID: 27861240 Free PMC article.
-
Semen IgM, IgG1, and IgG3 Differentially Associate With Pro-Inflammatory Cytokines in HIV-Infected Men.Front Immunol. 2019 Jan 23;9:3141. doi: 10.3389/fimmu.2018.03141. eCollection 2018. Front Immunol. 2019. PMID: 30728825 Free PMC article.
-
Clinical and Mucosal Immune Correlates of HIV-1 Semen Levels in Antiretroviral-Naive Men.Open Forum Infect Dis. 2017 Feb 21;4(2):ofx033. doi: 10.1093/ofid/ofx033. eCollection 2017 Spring. Open Forum Infect Dis. 2017. PMID: 28534034 Free PMC article.
-
Microbiome alterations in HIV infection a review.Cell Microbiol. 2016 May;18(5):645-51. doi: 10.1111/cmi.12588. Cell Microbiol. 2016. PMID: 26945815 Review.
-
[The male genital tract: A host for HIV].Gynecol Obstet Fertil. 2007 Dec;35(12):1245-50. doi: 10.1016/j.gyobfe.2007.09.017. Epub 2007 Nov 26. Gynecol Obstet Fertil. 2007. PMID: 18035579 Review. French.
Cited by
-
Predictors of Viral Load Status Over Time Among HIV Infected Adults Under HAART in Zewditu Memorial Hospital, Ethiopia: A Retrospective Study.HIV AIDS (Auckl). 2023 Feb 7;15:29-40. doi: 10.2147/HIV.S396030. eCollection 2023. HIV AIDS (Auckl). 2023. PMID: 36785672 Free PMC article.
-
The gut microbiome in human immunodeficiency virus infection.BMC Med. 2016 Jun 3;14(1):83. doi: 10.1186/s12916-016-0625-3. BMC Med. 2016. PMID: 27256449 Free PMC article. Review.
-
Seminal Plasma-Derived Extracellular-Vesicle Fractions from HIV-Infected Men Exhibit Unique MicroRNA Signatures and Induce a Proinflammatory Response in Cells Isolated from the Female Reproductive Tract.J Virol. 2020 Jul 30;94(16):e00525-20. doi: 10.1128/JVI.00525-20. Print 2020 Jul 30. J Virol. 2020. PMID: 32434889 Free PMC article.
-
Seminal Simian Immunodeficiency Virus in Chronically Infected Cynomolgus Macaques Is Dominated by Virus Originating from Multiple Genital Organs.J Virol. 2018 Jun 29;92(14):e00133-18. doi: 10.1128/JVI.00133-18. Print 2018 Jul 15. J Virol. 2018. PMID: 29720516 Free PMC article.
-
Semen Bacterial Concentrations and HIV-1 RNA Shedding Among HIV-1-Seropositive Kenyan Men.J Acquir Immune Defic Syndr. 2017 Mar 1;74(3):250-257. doi: 10.1097/QAI.0000000000001244. J Acquir Immune Defic Syndr. 2017. PMID: 27861240 Free PMC article.
References
-
- Kaul R, Pettengell C, Sheth PM, Sunderji S, Biringer A, et al. (2008) The genital tract immune milieu: an important determinant of HIV susceptibility and secondary transmission. Journal of reproductive immunology 77: 32–40. - PubMed
-
- Zhang H, Dornadula G, Beumont M, Livornese L Jr, Van Uitert B, et al. (1998) Human immunodeficiency virus type 1 in the semen of men receiving highly active antiretroviral therapy. The New England journal of medicine 339: 1803–1809. - PubMed
-
- Coombs RW, Speck CE, Hughes JP, Lee W, Sampoleo R, et al. (1998) Association between culturable human immunodeficiency virus type 1 (HIV-1) in semen and HIV-1 RNA levels in semen and blood: evidence for compartmentalization of HIV-1 between semen and blood. The Journal of infectious diseases 177: 320–330. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

