Effects of treatment with suppressive combination antiretroviral drug therapy and the histone deacetylase inhibitor suberoylanilide hydroxamic acid; (SAHA) on SIV-infected Chinese rhesus macaques
- PMID: 25033210
- PMCID: PMC4102539
- DOI: 10.1371/journal.pone.0102795
Effects of treatment with suppressive combination antiretroviral drug therapy and the histone deacetylase inhibitor suberoylanilide hydroxamic acid; (SAHA) on SIV-infected Chinese rhesus macaques
Abstract
Objectives: Viral reservoirs-persistent residual virus despite combination antiretroviral therapy (cART)-remain an obstacle to cure of HIV-1 infection. Difficulty studying reservoirs in patients underscores the need for animal models that mimics HIV infected humans on cART. We studied SIV-infected Chinese-origin rhesus macaques (Ch-RM) treated with intensive combination antiretroviral therapy (cART) and 3 weeks of treatment with the histone deacetyalse inhibitor, suberoylanilide hydroxamic acid (SAHA).
Methods: SIVmac251 infected Ch-RM received reverse transcriptase inhibitors PMPA and FTC and integrase inhibitor L-870812 beginning 7 weeks post infection. Integrase inhibitor L-900564 and boosted protease inhibitor treatment with Darunavir and Ritonavir were added later. cART was continued for 45 weeks, with daily SAHA administered for the last 3 weeks, followed by euthanasia/necropsy. Plasma viral RNA and cell/tissue-associated SIV gag RNA and DNA were quantified by qRT-PCR/qPCR, with flow cytometry monitoring changes in immune cell populations.
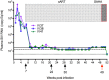
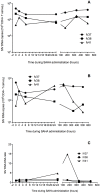
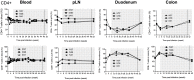
Results: Upon cART initiation, plasma viremia declined, remaining <30 SIV RNA copy Eq/ml during cART, with occasional blips. Decreased viral replication was associated with decreased immune activation and partial restoration of intestinal CD4+ T cells. SAHA was well tolerated but did not result in demonstrable treatment-associated changes in plasma or cell associated viral parameters.
Conclusions: The ability to achieve and sustain virological suppression makes cART-suppressed, SIV-infected Ch-RM a potentially useful model to evaluate interventions targeting residual virus. However, despite intensive cART over one year, persistent viral DNA and RNA remained in tissues of all three animals. While well tolerated, three weeks of SAHA treatment did not demonstrably impact viral RNA levels in plasma or tissues; perhaps reflecting dosing, sampling and assay limitations.
Conflict of interest statement
Figures




Similar articles
-
Effect of suberoylanilide hydroxamic acid (SAHA) administration on the residual virus pool in a model of combination antiretroviral therapy-mediated suppression in SIVmac239-infected indian rhesus macaques.Antimicrob Agents Chemother. 2014 Nov;58(11):6790-806. doi: 10.1128/AAC.03746-14. Epub 2014 Sep 2. Antimicrob Agents Chemother. 2014. PMID: 25182644 Free PMC article.
-
Evaluating the Intactness of Persistent Viral Genomes in Simian Immunodeficiency Virus-Infected Rhesus Macaques after Initiating Antiretroviral Therapy within One Year of Infection.J Virol. 2019 Dec 12;94(1):e01308-19. doi: 10.1128/JVI.01308-19. Print 2019 Dec 12. J Virol. 2019. PMID: 31597776 Free PMC article.
-
Effect of combination antiretroviral therapy on Chinese rhesus macaques of simian immunodeficiency virus infection.AIDS Res Hum Retroviruses. 2013 Nov;29(11):1465-74. doi: 10.1089/AID.2012.0378. Epub 2013 Mar 8. AIDS Res Hum Retroviruses. 2013. PMID: 23387294 Free PMC article.
-
Brain macrophages harbor latent, infectious simian immunodeficiency virus.AIDS. 2019 Dec 1;33 Suppl 2(Suppl 2):S181-S188. doi: 10.1097/QAD.0000000000002269. AIDS. 2019. PMID: 31789817 Free PMC article. Review.
-
Considerations in the development of nonhuman primate models of combination antiretroviral therapy for studies of AIDS virus suppression, residual virus, and curative strategies.Curr Opin HIV AIDS. 2013 Jul;8(4):262-72. doi: 10.1097/COH.0b013e328361cf40. Curr Opin HIV AIDS. 2013. PMID: 23698559 Free PMC article. Review.
Cited by
-
Can research at the end of life be a useful tool to advance HIV cure?AIDS. 2017 Jan 2;31(1):1-4. doi: 10.1097/QAD.0000000000001300. AIDS. 2017. PMID: 27755112 Free PMC article.
-
Persistence of SIV in the brain of SIV-infected Chinese rhesus macaques with or without antiretroviral therapy.J Neurovirol. 2018 Feb;24(1):62-74. doi: 10.1007/s13365-017-0594-0. Epub 2017 Nov 27. J Neurovirol. 2018. PMID: 29181724 Free PMC article.
-
Using animal models to overcome temporal, spatial and combinatorial challenges in HIV persistence research.J Transl Med. 2016 Feb 9;14:44. doi: 10.1186/s12967-016-0807-y. J Transl Med. 2016. PMID: 26861779 Free PMC article. Review.
-
Reactivation of simian immunodeficiency virus reservoirs in the brain of virally suppressed macaques.AIDS. 2017 Jan 2;31(1):5-14. doi: 10.1097/QAD.0000000000001267. AIDS. 2017. PMID: 27898590 Free PMC article.
-
Genomic Tools for the Use of Nonhuman Primates in Translational Research.ILAR J. 2017 Jul 1;58(1):59-68. doi: 10.1093/ilar/ilw042. ILAR J. 2017. PMID: 28838069 Free PMC article. Review.
References
-
- Zink MC, Brice AK, Kelly KM, Queen SE, Gama L, et al. (2010) Simian immunodeficiency virus-infected macaques treated with highly active antiretroviral therapy have reduced central nervous system viral replication and inflammation but persistence of viral DNA. J Infect Dis 202: 161–170. - PMC - PubMed
-
- Poles MA, Boscardin WJ, Elliott J, Taing P, Fuerst MM, et al. (2006) Lack of decay of HIV-1 in gut-associated lymphoid tissue reservoirs in maximally suppressed individuals. J Acquir Immune Defic Syndr 43: 65–68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

