Comparative proteomic study reveals 17β-HSD13 as a pathogenic protein in nonalcoholic fatty liver disease
- PMID: 25028495
- PMCID: PMC4128098
- DOI: 10.1073/pnas.1410741111
Comparative proteomic study reveals 17β-HSD13 as a pathogenic protein in nonalcoholic fatty liver disease
Abstract
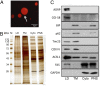
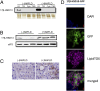
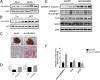
Nonalcoholic fatty liver disease (NAFLD) is characterized by a massive accumulation of lipid droplets (LDs). The aim of this study was to determine the function of 17β-hydroxysteroid dehydrogenase-13 (17β-HSD13), one of our newly identified LD-associated proteins in human subjects with normal liver histology and simple steatosis, in NAFLD development. LDs were isolated from 21 human liver biopsies, including 9 cases with normal liver histology (group 1) and 12 cases with simple steatosis (group 2). A complete set of LD-associated proteins from three liver samples of group 1 or group 2 were determined by 2D LC-MS/MS. By comparing the LD-associated protein profiles between subjects with or without NAFLD, 54 up-regulated and 35 down-regulated LD-associated proteins were found in NAFLD patients. Among them, 17β-HSD13 represents a previously unidentified LD-associated protein with a significant up-regulation in NAFLD. Because the 17β-HSD family plays an important role in lipid metabolism, 17β-HSD13 was selected for validating the proteomic findings and exploring its role in the pathogenesis of NAFLD. Increased hepatic 17β-HSD13 and its LD surface location were confirmed in db/db (diabetic) and high-fat diet-fed mice. Adenovirus-mediated hepatic overexpression of human 17β-HSD13 induced a fatty liver phenotype in C57BL/6 mice, with a significant increase in mature sterol regulatory element-binding protein 1 and fatty acid synthase levels. The present study reports an extensive set of human liver LD proteins and an array of proteins differentially expressed in human NAFLD. We also identified 17β-HSD13 as a pathogenic protein in the development of NAFLD.
Keywords: HSDI7β13; SCDR9; lipogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Liver X receptor α induces 17β-hydroxysteroid dehydrogenase-13 expression through SREBP-1c.Am J Physiol Endocrinol Metab. 2017 Apr 1;312(4):E357-E367. doi: 10.1152/ajpendo.00310.2016. Epub 2017 Mar 7. Am J Physiol Endocrinol Metab. 2017. PMID: 28270440
-
Omic studies reveal the pathogenic lipid droplet proteins in non-alcoholic fatty liver disease.Protein Cell. 2017 Jan;8(1):4-13. doi: 10.1007/s13238-016-0327-9. Epub 2016 Oct 18. Protein Cell. 2017. PMID: 27757845 Free PMC article. Review.
-
The role of hepassocin in the development of non-alcoholic fatty liver disease.J Hepatol. 2013 Nov;59(5):1065-72. doi: 10.1016/j.jhep.2013.06.004. Epub 2013 Jun 18. J Hepatol. 2013. PMID: 23792031
-
Thrombospondin 1 improves hepatic steatosis in diet-induced insulin-resistant mice and is associated with hepatic fat content in humans.EBioMedicine. 2020 Jul;57:102849. doi: 10.1016/j.ebiom.2020.102849. Epub 2020 Jun 21. EBioMedicine. 2020. PMID: 32580141 Free PMC article.
-
Role of HSD17B13 in the liver physiology and pathophysiology.Mol Cell Endocrinol. 2019 Jun 1;489:119-125. doi: 10.1016/j.mce.2018.10.014. Epub 2018 Oct 24. Mol Cell Endocrinol. 2019. PMID: 30365983 Review.
Cited by
-
Identification of prognostic biomarkers for patients with hepatocellular carcinoma after hepatectomy.Oncol Rep. 2019 Mar;41(3):1586-1602. doi: 10.3892/or.2019.6953. Epub 2019 Jan 3. Oncol Rep. 2019. PMID: 30628708 Free PMC article.
-
Genetics Is of the Essence to Face NAFLD.Biomedicines. 2021 Sep 30;9(10):1359. doi: 10.3390/biomedicines9101359. Biomedicines. 2021. PMID: 34680476 Free PMC article. Review.
-
Review article: the emerging role of genetics in precision medicine for patients with non-alcoholic steatohepatitis.Aliment Pharmacol Ther. 2020 Jun;51(12):1305-1320. doi: 10.1111/apt.15738. Epub 2020 May 7. Aliment Pharmacol Ther. 2020. PMID: 32383295 Free PMC article. Review.
-
Is HSD17B13 Genetic Variant a Protector for Liver Dysfunction? Future Perspective as a Potential Therapeutic Target.J Pers Med. 2021 Jun 30;11(7):619. doi: 10.3390/jpm11070619. J Pers Med. 2021. PMID: 34208839 Free PMC article. Review.
-
Modelling human liver fibrosis in the context of non-alcoholic steatohepatitis using a microphysiological system.Commun Biol. 2021 Sep 15;4(1):1080. doi: 10.1038/s42003-021-02616-x. Commun Biol. 2021. PMID: 34526653 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

