The tumor necrosis factor alpha-induced protein 3 (TNFAIP3, A20) imposes a brake on antitumor activity of CD8 T cells
- PMID: 25024217
- PMCID: PMC4121810
- DOI: 10.1073/pnas.1406259111
The tumor necrosis factor alpha-induced protein 3 (TNFAIP3, A20) imposes a brake on antitumor activity of CD8 T cells
Abstract
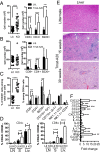
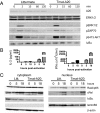
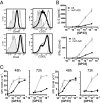
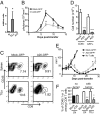
The transcription factor NF-κB is central to inflammatory signaling and activation of innate and adaptive immune responses. Activation of the NF-κB pathway is tightly controlled by several negative feedback mechanisms, including A20, an ubiquitin-modifying enzyme encoded by the tnfaip3 gene. Mice with selective deletion of A20 in myeloid, dendritic, or B cells recapitulate some human inflammatory pathology. As we observed high expression of A20 transcripts in dysfunctional CD8 T cells in an autochthonous melanoma, we analyzed the role of A20 in regulation of CD8 T-cell functions, using mice in which A20 was selectively deleted in mature conventional T cells. These mice developed lymphadenopathy and some organ infiltration by T cells but no splenomegaly and no detectable pathology. A20-deleted CD8 T cells had increased sensitivity to antigen stimulation with production of large amounts of IL-2 and IFNγ, correlated with sustained nuclear expression of NF-κB components reticuloendotheliosis oncogene c-Rel and p65. Overexpression of A20 by retroviral transduction of CD8 T cells dampened their intratumor accumulation and antitumor activity. In contrast, relief from the A20 brake in NF-κB activation in adoptively transferred antitumor CD8 T cells led to improved control of melanoma growth. Tumor-infiltrating A20-deleted CD8 T cells had enhanced production of IFNγ and TNFα and reduced expression of the inhibitory receptor programmed cell death 1. As manipulation of A20 expression in CD8 T cells did not result in pathologic manifestations in the mice, we propose it as a candidate to be targeted to increase antitumor efficiency of adoptive T-cell immunotherapy.
Keywords: T-cell activation; inflammation; tumor immunity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Keratinocyte-specific ablation of the NF-κB regulatory protein A20 (TNFAIP3) reveals a role in the control of epidermal homeostasis.Cell Death Differ. 2011 Dec;18(12):1845-53. doi: 10.1038/cdd.2011.55. Epub 2011 May 13. Cell Death Differ. 2011. PMID: 21566665 Free PMC article.
-
B cells lacking the tumor suppressor TNFAIP3/A20 display impaired differentiation and hyperactivation and cause inflammation and autoimmunity in aged mice.Blood. 2011 Feb 17;117(7):2227-36. doi: 10.1182/blood-2010-09-306019. Epub 2010 Nov 18. Blood. 2011. PMID: 21088135
-
Vitamin E γ-Tocotrienol Inhibits Cytokine-Stimulated NF-κB Activation by Induction of Anti-Inflammatory A20 via Stress Adaptive Response Due to Modulation of Sphingolipids.J Immunol. 2015 Jul 1;195(1):126-33. doi: 10.4049/jimmunol.1403149. Epub 2015 May 22. J Immunol. 2015. PMID: 26002975 Free PMC article.
-
A20/Tumor Necrosis Factor α-Induced Protein 3 in Immune Cells Controls Development of Autoinflammation and Autoimmunity: Lessons from Mouse Models.Front Immunol. 2018 Feb 21;9:104. doi: 10.3389/fimmu.2018.00104. eCollection 2018. Front Immunol. 2018. PMID: 29515565 Free PMC article. Review.
-
The ubiquitin-editing enzyme A20 (TNFAIP3) is a central regulator of immunopathology.Trends Immunol. 2009 Aug;30(8):383-91. doi: 10.1016/j.it.2009.05.007. Epub 2009 Jul 28. Trends Immunol. 2009. PMID: 19643665 Review.
Cited by
-
A20 at the Crossroads of Cell Death, Inflammation, and Autoimmunity.Cold Spring Harb Perspect Biol. 2020 Jan 2;12(1):a036418. doi: 10.1101/cshperspect.a036418. Cold Spring Harb Perspect Biol. 2020. PMID: 31427375 Free PMC article. Review.
-
Predicting single-cell gene expression profiles of imaging flow cytometry data with machine learning.Nucleic Acids Res. 2020 Nov 18;48(20):11335-11346. doi: 10.1093/nar/gkaa926. Nucleic Acids Res. 2020. PMID: 33119742 Free PMC article.
-
IFNγ producing CD8+ T cells modified to resist major immune checkpoints induce regression of MHC class I-deficient melanomas.Oncoimmunology. 2015 Mar 6;4(2):e974959. doi: 10.4161/2162402X.2014.974959. eCollection 2015 Feb. Oncoimmunology. 2015. PMID: 25949872 Free PMC article.
-
NF-κB regulated expression of A20 controls IKK dependent repression of RIPK1 induced cell death in activated T cells.Cell Death Differ. 2025 Feb;32(2):256-270. doi: 10.1038/s41418-024-01383-6. Epub 2024 Sep 26. Cell Death Differ. 2025. PMID: 39327505 Free PMC article.
-
Regulatory circuits of T cell function in cancer.Nat Rev Immunol. 2016 Oct;16(10):599-611. doi: 10.1038/nri.2016.80. Epub 2016 Aug 16. Nat Rev Immunol. 2016. PMID: 27526640 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

