Fasting increases human skeletal muscle net phenylalanine release and this is associated with decreased mTOR signaling
- PMID: 25020061
- PMCID: PMC4096723
- DOI: 10.1371/journal.pone.0102031
Fasting increases human skeletal muscle net phenylalanine release and this is associated with decreased mTOR signaling
Abstract
Aim: Fasting is characterised by profound changes in energy metabolism including progressive loss of body proteins. The underlying mechanisms are however unknown and we therefore determined the effects of a 72-hour-fast on human skeletal muscle protein metabolism and activation of mammalian target of rapamycin (mTOR), a key regulator of cell growth.
Methods: Eight healthy male volunteers were studied twice: in the postabsorptive state and following 72 hours of fasting. Regional muscle amino acid kinetics was measured in the forearm using amino acid tracers. Signaling to protein synthesis and breakdown were assessed in skeletal muscle biopsies obtained during non-insulin and insulin stimulated conditions on both examination days.
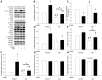
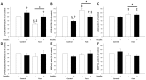
Results: Fasting significantly increased forearm net phenylalanine release and tended to decrease phenylalanine rate of disappearance. mTOR phosphorylation was decreased by ∼50% following fasting, together with reduced downstream phosphorylation of 4EBP1, ULK1 and rpS6. In addition, the insulin stimulated increase in mTOR and rpS6 phosphorylation was significantly reduced after fasting indicating insulin resistance in this part of the signaling pathway. Autophagy initiation is in part regulated by mTOR through ULK1 and fasting increased expression of the autophagic marker LC3B-II by ∼30%. p62 is degraded during autophagy but was increased by ∼10% during fasting making interpretation of autophagic flux problematic. MAFbx and MURF1 ubiquitin ligases remained unaltered after fasting indicating no change in protesomal protein degradation.
Conclusions: Our results show that during fasting increased net phenylalanine release in skeletal muscle is associated to reduced mTOR activation and concomitant decreased downstream signaling to cell growth.
Conflict of interest statement
Figures
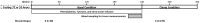

Similar articles
-
Amino acid supplementation is anabolic during the acute phase of endotoxin-induced inflammation: A human randomized crossover trial.Clin Nutr. 2016 Apr;35(2):322-330. doi: 10.1016/j.clnu.2015.03.021. Epub 2015 Apr 9. Clin Nutr. 2016. PMID: 25896101 Clinical Trial.
-
Regulation of Akt-mTOR, ubiquitin-proteasome and autophagy-lysosome pathways in response to formoterol administration in rat skeletal muscle.Int J Biochem Cell Biol. 2013 Nov;45(11):2444-55. doi: 10.1016/j.biocel.2013.07.019. Epub 2013 Aug 2. Int J Biochem Cell Biol. 2013. PMID: 23916784
-
Differential regulation of lipid and protein metabolism in obese vs. lean subjects before and after a 72-h fast.Am J Physiol Endocrinol Metab. 2016 Jul 1;311(1):E224-35. doi: 10.1152/ajpendo.00464.2015. Epub 2016 May 31. Am J Physiol Endocrinol Metab. 2016. PMID: 27245338
-
PI3 kinase regulation of skeletal muscle hypertrophy and atrophy.Curr Top Microbiol Immunol. 2010;346:267-78. doi: 10.1007/82_2010_78. Curr Top Microbiol Immunol. 2010. PMID: 20593312 Review.
-
mTor signaling in skeletal muscle during sepsis and inflammation: where does it all go wrong?Physiology (Bethesda). 2011 Apr;26(2):83-96. doi: 10.1152/physiol.00044.2010. Physiology (Bethesda). 2011. PMID: 21487027 Free PMC article. Review.
Cited by
-
Does Timing Matter? A Narrative Review of Intermittent Fasting Variants and Their Effects on Bodyweight and Body Composition.Nutrients. 2022 Nov 25;14(23):5022. doi: 10.3390/nu14235022. Nutrients. 2022. PMID: 36501050 Free PMC article. Review.
-
Molecular and cellular adaptations to exercise training in skeletal muscle from cancer patients treated with chemotherapy.J Cancer Res Clin Oncol. 2019 Jun;145(6):1449-1460. doi: 10.1007/s00432-019-02911-5. Epub 2019 Apr 9. J Cancer Res Clin Oncol. 2019. PMID: 30968255
-
Effects of beta-hydroxy-beta-methylbutyrate (HMB) on the expression of ubiquitin ligases, protein synthesis pathways and contractile function in extensor digitorum longus (EDL) of fed and fasting rats.J Physiol Sci. 2018 Mar;68(2):165-174. doi: 10.1007/s12576-016-0520-x. Epub 2017 Jan 12. J Physiol Sci. 2018. PMID: 28083734 Free PMC article.
-
Compromised autophagy and mitophagy in brain ageing and Alzheimer's diseases.Aging Brain. 2022 Nov 24;2:100056. doi: 10.1016/j.nbas.2022.100056. eCollection 2022. Aging Brain. 2022. PMID: 36908880 Free PMC article. Review.
-
Combined Aerobic Exercise with Intermittent Fasting Is Effective for Reducing mTOR and Bcl-2 Levels in Obese Females.Sports (Basel). 2024 Apr 25;12(5):116. doi: 10.3390/sports12050116. Sports (Basel). 2024. PMID: 38786985 Free PMC article.
References
-
- Thomson TJ, Runcie J, Miller V (1966) Treatment of obesity by total fasting for up to 249 days. Lancet 2: 992–996. - PubMed
-
- Cahill GF Jr (1970) Starvation in man. N Engl J Med 282: 668–675. - PubMed
-
- Owen OE, Smalley KJ, D'Alessio DA, Mozzoli MA, Dawson EK (1998) Protein, fat, and carbohydrate requirements during starvation: anaplerosis and cataplerosis. Am J Clin Nutr 68: 12–34. - PubMed
-
- Fryburg DA, Barrett EJ, Louard RJ, Gelfand RA (1990) Effect of starvation on human muscle protein metabolism and its response to insulin. Am J Physiol 259: E477–482. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

