Macrophage PTEN regulates expression and secretion of arginase I modulating innate and adaptive immune responses
- PMID: 25015834
- PMCID: PMC4120896
- DOI: 10.4049/jimmunol.1302167
Macrophage PTEN regulates expression and secretion of arginase I modulating innate and adaptive immune responses
Erratum in
- J Immunol. 2014 Nov 15;193(10):5350. Dohnal, Alexander B [Corrected to Dohnal, Alexander M]
Abstract
The activation of innate immune cells triggers numerous intracellular signaling pathways, which require tight control to mount an adequate immune response. The PI3K signaling pathway is intricately involved in innate immunity, and its activation dampens the expression and release of proinflammatory cytokines in myeloid cells. These signaling processes are strictly regulated by the PI3K antagonist, the lipid phosphatase, PTEN, a known tumor suppressor. Importantly, PTEN is responsible for the elevated production of cytokines such as IL-6 in response to TLR agonists, and deletion of PTEN results in diminished inflammatory responses. However, the mechanisms by which PI3K negatively regulates TLR signaling are only partially resolved. We observed that Arginase I expression and secretion were markedly induced by PTEN deletion, suggesting PTEN(-/-) macrophages were alternatively activated. This was mediated by increased expression and activation of the transcription factors C/EBPβ and STAT3. Genetic and pharmacologic experimental approaches in vitro, as well as in vivo autoimmunity models, provide convincing evidence that PI3K/PTEN-regulated extracellular Arginase I acts as a paracrine regulator of inflammation and immunity.
Copyright © 2014 by The American Association of Immunologists, Inc.
Figures



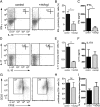
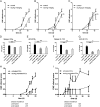
Similar articles
-
HO-1-STAT3 axis in mouse liver ischemia/reperfusion injury: regulation of TLR4 innate responses through PI3K/PTEN signaling.J Hepatol. 2012 Feb;56(2):359-66. doi: 10.1016/j.jhep.2011.05.023. Epub 2011 Jul 12. J Hepatol. 2012. PMID: 21756853 Free PMC article.
-
The role of JAK-3 in regulating TLR-mediated inflammatory cytokine production in innate immune cells.J Immunol. 2013 Aug 1;191(3):1164-74. doi: 10.4049/jimmunol.1203084. Epub 2013 Jun 24. J Immunol. 2013. PMID: 23797672 Free PMC article.
-
The inositol 3-phosphatase PTEN negatively regulates Fc gamma receptor signaling, but supports Toll-like receptor 4 signaling in murine peritoneal macrophages.J Immunol. 2004 Apr 15;172(8):4851-7. doi: 10.4049/jimmunol.172.8.4851. J Immunol. 2004. PMID: 15067063
-
Recent advances in the genetic analysis of PTEN and PI3K innate immune properties.Immunobiology. 2008;213(9-10):759-65. doi: 10.1016/j.imbio.2008.07.028. Epub 2008 Sep 11. Immunobiology. 2008. PMID: 18926291 Review.
-
The Role of PTEN in Innate and Adaptive Immunity.Cold Spring Harb Perspect Med. 2019 Dec 2;9(12):a036996. doi: 10.1101/cshperspect.a036996. Cold Spring Harb Perspect Med. 2019. PMID: 31501268 Free PMC article. Review.
Cited by
-
Oral activity of a nature-derived cyclic peptide for the treatment of multiple sclerosis.Proc Natl Acad Sci U S A. 2016 Apr 12;113(15):3960-5. doi: 10.1073/pnas.1519960113. Epub 2016 Mar 28. Proc Natl Acad Sci U S A. 2016. PMID: 27035952 Free PMC article.
-
Benznidazole Anti-Inflammatory Effects in Murine Cardiomyocytes and Macrophages Are Mediated by Class I PI3Kδ.Front Immunol. 2021 Dec 2;12:782891. doi: 10.3389/fimmu.2021.782891. eCollection 2021. Front Immunol. 2021. PMID: 34925364 Free PMC article.
-
Exosomal miRNA-107 induces myeloid-derived suppressor cell expansion in gastric cancer.Cancer Manag Res. 2019 May 6;11:4023-4040. doi: 10.2147/CMAR.S198886. eCollection 2019. Cancer Manag Res. 2019. PMID: 31190980 Free PMC article.
-
Granulosa cell-derived miR-379-5p regulates macrophage polarization in polycystic ovarian syndrome.Front Immunol. 2023 Mar 24;14:1104550. doi: 10.3389/fimmu.2023.1104550. eCollection 2023. Front Immunol. 2023. PMID: 37033997 Free PMC article.
-
Tumor-derived exosomes in the regulation of macrophage polarization.Inflamm Res. 2020 May;69(5):435-451. doi: 10.1007/s00011-020-01318-0. Epub 2020 Mar 11. Inflamm Res. 2020. PMID: 32162012 Review.
References
-
- Engelman J. A., Luo J., Cantley L. C. 2006. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 7: 606–619 - PubMed
-
- Ferguson G. J., Milne L., Kulkarni S., Sasaki T., Walker S., Andrews S., Crabbe T., Finan P., Jones G., Jackson S., et al. 2007. PI(3)Kgamma has an important context-dependent role in neutrophil chemokinesis. Nat. Cell Biol. 9: 86–91 - PubMed
-
- Heit B., Robbins S. M., Downey C. M., Guan Z., Colarusso P., Miller B. J., Jirik F. R., Kubes P. 2008. PTEN functions to ‘prioritize’ chemotactic cues and prevent ‘distraction’ in migrating neutrophils. Nat. Immunol. 9: 743–752 - PubMed
-
- Aksoy E., Taboubi S., Torres D., Delbauve S., Hachani A., Whitehead M. A., Pearce W. P., Berenjeno I. M., Nock G., Filloux A., et al. 2012. The p110δ isoform of the kinase PI(3)K controls the subcellular compartmentalization of TLR4 signaling and protects from endotoxic shock. [Published erratum appears in 2013 Nat. Immunol. 14: 877.] Nat. Immunol. 13: 1045–1054 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

