Diverse specificities, phenotypes, and antiviral activities of cytomegalovirus-specific CD8+ T cells
- PMID: 25008941
- PMCID: PMC4178885
- DOI: 10.1128/JVI.01477-14
Diverse specificities, phenotypes, and antiviral activities of cytomegalovirus-specific CD8+ T cells
Abstract
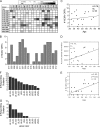
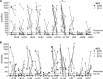
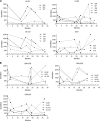
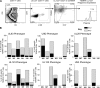
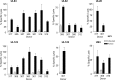
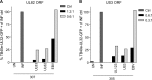
CD8(+) T cells specific for pp65, IE1, and IE2 are present at high frequencies in human cytomegalovirus (HCMV)-seropositive individuals, and these have been shown to have phenotypes associated with terminal differentiation, as well as both cytokine and proliferative dysfunctions, especially in the elderly. However, more recently, T cell responses to many other HCMV proteins have been described, but little is known about their phenotypes and functions. Consequently, in this study, we chose to determine the diversity of HCMV-specific CD8(+) T cell responses to the products of 11 HCMV open reading frames (ORFs) in a cohort of donors aged 20 to 80 years old as well as the ability of the T cells to secrete gamma interferon (IFN-γ). Finally, we also tested their functional antiviral capacity using a novel viral dissemination assay. We identified substantial CD8(+) T cell responses by IFN-γ enzyme-linked immunospot (ELISPOT) assays to all 11 of these HCMV proteins, and across the cohort, individuals displayed a range of responses, from tightly focused to highly diverse, which were stable over time. CD8(+) T cell responses to the HCMV ORFs were highly differentiated and predominantly CD45RA(+), CD57(+), and CD28(-), across the cohort. These highly differentiated cells had the ability to inhibit viral spread even following direct ex vivo isolation. Taken together, our data argue that HCMV-specific CD8(+) T cells have effective antiviral activity irrespective of the viral protein recognized across the whole cohort and despite viral immune evasion.
Importance: Human cytomegalovirus (HCMV) is normally carried without clinical symptoms and is widely prevalent in the population; however, it often causes severe clinical disease in individuals with compromised immune responses. HCMV is never cleared after primary infection but persists in the host for life. In HCMV carriers, the immune response to HCMV includes large numbers of virus-specific immune cells, and the virus has evolved many mechanisms to evade the immune response. While this immune response seems to protect healthy people from subsequent disease, the virus is never eliminated. It has been suggested that this continuous surveillance by the immune system may have deleterious effects in later life. The study presented in this paper examined immune responses from a cohort of donors and shows that these immune cells are effective at controlling the virus and can overcome the virus' lytic cycle immune evasion mechanisms.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures


Similar articles
-
Human Cytomegalovirus (HCMV)-Specific CD4+ T Cells Are Polyfunctional and Can Respond to HCMV-Infected Dendritic Cells In Vitro.J Virol. 2017 Feb 28;91(6):e02128-16. doi: 10.1128/JVI.02128-16. Print 2017 Mar 15. J Virol. 2017. PMID: 28053099 Free PMC article.
-
Human cytomegalovirus proteins pp65 and immediate early protein 1 are common targets for CD8+ T cell responses in children with congenital or postnatal human cytomegalovirus infection.J Immunol. 2004 Feb 15;172(4):2256-64. doi: 10.4049/jimmunol.172.4.2256. J Immunol. 2004. PMID: 14764694
-
Repertoire, diversity, and differentiation of specific CD8 T cells are associated with immune protection against human cytomegalovirus disease.J Exp Med. 2005 Jun 20;201(12):1999-2010. doi: 10.1084/jem.20042408. J Exp Med. 2005. PMID: 15967826 Free PMC article.
-
Molecular characterization of HCMV-specific immune responses: Parallels between CD8(+) T cells, CD4(+) T cells, and NK cells.Eur J Immunol. 2015 Sep;45(9):2433-45. doi: 10.1002/eji.201545495. Epub 2015 Aug 24. Eur J Immunol. 2015. PMID: 26228786 Review.
-
Cytomegalovirus and the aging population.Drugs Aging. 2001;18(12):927-33. doi: 10.2165/00002512-200118120-00004. Drugs Aging. 2001. PMID: 11888347 Review.
Cited by
-
In Vitro Profiling of Commonly Used Post-transplant Immunosuppressants Reveals Distinct Impact on Antiviral T-cell Immunity Towards CMV.Transpl Int. 2024 Apr 9;37:12720. doi: 10.3389/ti.2024.12720. eCollection 2024. Transpl Int. 2024. PMID: 38655204 Free PMC article.
-
Applications of Anti-Cytomegalovirus T Cells for Cancer (Immuno)Therapy.Cancers (Basel). 2023 Jul 25;15(15):3767. doi: 10.3390/cancers15153767. Cancers (Basel). 2023. PMID: 37568582 Free PMC article. Review.
-
Hematopoietic stem cell donor vaccination with cytomegalovirus triplex augments frequencies of functional and durable cytomegalovirus-specific T cells in the recipient: A novel strategy to limit antiviral prophylaxis.Am J Hematol. 2023 Apr;98(4):588-597. doi: 10.1002/ajh.26824. Epub 2023 Jan 11. Am J Hematol. 2023. PMID: 36594185 Free PMC article.
-
HCMV carriage in the elderly diminishes anti-viral functionality of the adaptive immune response resulting in virus replication at peripheral sites.Front Immunol. 2022 Dec 15;13:1083230. doi: 10.3389/fimmu.2022.1083230. eCollection 2022. Front Immunol. 2022. PMID: 36591233 Free PMC article.
-
IL-10-Secreting CD8+ T Cells Specific for Human Cytomegalovirus (HCMV): Generation, Maintenance and Phenotype.Pathogens. 2022 Dec 13;11(12):1530. doi: 10.3390/pathogens11121530. Pathogens. 2022. PMID: 36558866 Free PMC article.
References
-
- Walter EA, Greenberg PD, Gilbert MJ, Finch RJ, Watanabe KS, Thomas ED, Riddell SR. 1995. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N. Engl. J. Med. 333:1038–1044. 10.1056/NEJM199510193331603 - DOI - PubMed
-
- Einsele H, Roosnek E, Rufer N, Sinzger C, Riegler S, Loffler J, Grigoleit U, Moris A, Rammensee HG, Kanz L, Kleihauer A, Frank F, Jahn G, Hebart H. 2002. Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood 99:3916–3922. 10.1182/blood.V99.11.3916 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

