Feasibility study of personalized peptide vaccination for metastatic recurrent triple-negative breast cancer patients
- PMID: 24992895
- PMCID: PMC4227005
- DOI: 10.1186/bcr3685
Feasibility study of personalized peptide vaccination for metastatic recurrent triple-negative breast cancer patients
Abstract
Introduction: Since treatment modalities for metastatic recurrent triple-negative breast cancer (mrTNBC) are limited, a novel treatment approach including immunotherapy is required. We have developed a novel regimen of personalized peptide vaccination (PPV), in which vaccine antigens are individually selected from a pool of different peptide candidates based on the pre-existing host immunity. Herein we conducted a phase II study of PPV for metastatic recurrent breast cancer patients to investigate the feasibility of PPV for mrTNBC.
Methods: Seventy-nine patients with metastatic recurrent breast cancer who had metastases and had failed standard chemotherapy and/or hormonal therapy were enrolled. They were subgrouped as the mrTNBC group (n = 18), the luminal/human epidermal growth factor receptor 2 (HER2)-negative group (n = 41) and the HER2-positive group (n = 18), while the remaining two patients had not been investigated. A maximum of four human leukocyte antigen (HLA)-matched peptides showing higher peptide-specific immunoglobulin G (IgG) responses in pre-vaccination plasma were selected from 31 pooled peptide candidates applicable for the four HLA-IA phenotypes (HLA-A2, -A24, or -A26 types, or HLA-A3 supertypes), and were subcutaneously administered weekly for 6 weeks and bi-weekly thereafter. Measurement of peptide-specific cytotoxic T lymphocyte (CTL) and IgG responses along with other laboratory analyses were conducted before and after vaccination.
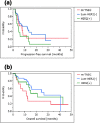
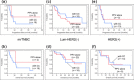
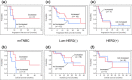
Results: No severe adverse events associated with PPV were observed in any of the enrolled patients. Boosting of CTL and/or IgG responses was observed in most of the patients after vaccination, irrespective of the breast cancer subtypes. There were three complete response cases (1 mrTNBC and 2 luminal/HER2-negative types) and six partial response cases (1 mrTNBC and 5 luminal/HER2-negative types). The median progression-free survival time and median overall survival time of mrTNBC patients were 7.5 and 11.1 months, while those of luminal/HER2-negative patients were 12.2 and 26.5 months, and those of HER2-positive patients were 4.5 and 14.9 months, respectively.
Conclusions: PPV could be feasible for mrTNBC patients because of the safety, immune responses, and possible clinical benefits.
Clinical trial registration number: UMIN000001844 (Registration Date: April 5, 2009).
Figures


Similar articles
-
Phase II study of personalized peptide vaccination for refractory bone and soft tissue sarcoma patients.Cancer Sci. 2013 Oct;104(10):1285-94. doi: 10.1111/cas.12226. Epub 2013 Aug 6. Cancer Sci. 2013. PMID: 23829867 Free PMC article. Clinical Trial.
-
Early phase II study of mixed 19-peptide vaccine monotherapy for refractory triple-negative breast cancer.Cancer Sci. 2020 Aug;111(8):2760-2769. doi: 10.1111/cas.14510. Epub 2020 Jun 25. Cancer Sci. 2020. PMID: 32495455 Free PMC article. Clinical Trial.
-
Immunological evaluation of peptide vaccination for cancer patients with the HLA-A26 allele.Cancer Sci. 2015 Oct;106(10):1257-63. doi: 10.1111/cas.12757. Epub 2015 Sep 25. Cancer Sci. 2015. PMID: 26212219 Free PMC article. Clinical Trial.
-
Prospect and progress of personalized peptide vaccinations for advanced cancers.Expert Opin Biol Ther. 2016;16(5):689-98. doi: 10.1517/14712598.2016.1161752. Epub 2016 Mar 21. Expert Opin Biol Ther. 2016. PMID: 26938083 Review.
-
Personalized peptide vaccination: a new approach for advanced cancer as therapeutic cancer vaccine.Cancer Immunol Immunother. 2013 May;62(5):919-29. doi: 10.1007/s00262-012-1379-1. Epub 2012 Nov 30. Cancer Immunol Immunother. 2013. PMID: 23197273 Free PMC article. Review.
Cited by
-
Trial Watch: Peptide-based anticancer vaccines.Oncoimmunology. 2015 Jan 9;4(4):e974411. doi: 10.4161/2162402X.2014.974411. eCollection 2015 Apr. Oncoimmunology. 2015. PMID: 26137405 Free PMC article. Review.
-
Immunotherapeutic interventions of Triple Negative Breast Cancer.J Transl Med. 2018 May 30;16(1):147. doi: 10.1186/s12967-018-1514-7. J Transl Med. 2018. PMID: 29848327 Free PMC article. Review.
-
Advances in immunotherapy for triple-negative breast cancer.Mol Cancer. 2023 Sep 2;22(1):145. doi: 10.1186/s12943-023-01850-7. Mol Cancer. 2023. PMID: 37660039 Free PMC article. Review.
-
The Clinical Significance of CD169-Positive Lymph Node Macrophage in Patients with Breast Cancer.PLoS One. 2016 Nov 18;11(11):e0166680. doi: 10.1371/journal.pone.0166680. eCollection 2016. PLoS One. 2016. PMID: 27861544 Free PMC article.
-
Immunotherapy in triple negative breast cancer: beyond checkpoint inhibitors.NPJ Breast Cancer. 2022 Nov 9;8(1):121. doi: 10.1038/s41523-022-00486-y. NPJ Breast Cancer. 2022. PMID: 36351947 Free PMC article. Review.
References
-
- Gradishar WJ, Krasnojon D, Cheporov S, Makhson AN, Manikhas GM, Clawson A, Bhar P. Significantly longer progression-free survival with nab-paclitaxel compared with docetaxel as first-line therapy for metastatic breast cancer. J Clin Oncol. 2009;27:3611–3619. doi: 10.1200/JCO.2008.18.5397. - DOI - PubMed
-
- Cortes J, O'Shaughnessy J, Loesch D, Blum JL, Vahdat LT, Petrakova K, Chollet P, Manikas A, Diéras V, Delozier T, Vladimirov V, Cardoso F, Koh H, Bougnoux P, Dutcus CE, Seegobin S, Mir D, Meneses N, Wanders J, Twelves C. Eribulin monotherapy versus treatment of physician's choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet. 2011;377:914–923. doi: 10.1016/S0140-6736(11)60070-6. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

