Host-directed therapy of tuberculosis based on interleukin-1 and type I interferon crosstalk
- PMID: 24990750
- PMCID: PMC4809146
- DOI: 10.1038/nature13489
Host-directed therapy of tuberculosis based on interleukin-1 and type I interferon crosstalk
Abstract
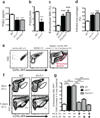
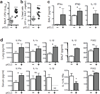
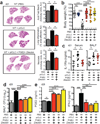
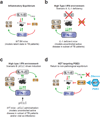
Tuberculosis remains second only to HIV/AIDS as the leading cause of mortality worldwide due to a single infectious agent. Despite chemotherapy, the global tuberculosis epidemic has intensified because of HIV co-infection, the lack of an effective vaccine and the emergence of multi-drug-resistant bacteria. Alternative host-directed strategies could be exploited to improve treatment efficacy and outcome, contain drug-resistant strains and reduce disease severity and mortality. The innate inflammatory response elicited by Mycobacterium tuberculosis (Mtb) represents a logical host target. Here we demonstrate that interleukin-1 (IL-1) confers host resistance through the induction of eicosanoids that limit excessive type I interferon (IFN) production and foster bacterial containment. We further show that, in infected mice and patients, reduced IL-1 responses and/or excessive type I IFN induction are linked to an eicosanoid imbalance associated with disease exacerbation. Host-directed immunotherapy with clinically approved drugs that augment prostaglandin E2 levels in these settings prevented acute mortality of Mtb-infected mice. Thus, IL-1 and type I IFNs represent two major counter-regulatory classes of inflammatory cytokines that control the outcome of Mtb infection and are functionally linked via eicosanoids. Our findings establish proof of concept for host-directed treatment strategies that manipulate the host eicosanoid network and represent feasible alternatives to conventional chemotherapy.
Figures

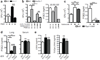


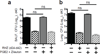

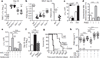
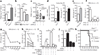


Comment in
-
Immunology: Fixing the odds against tuberculosis.Nature. 2014 Jul 3;511(7507):39-40. doi: 10.1038/nature13512. Epub 2014 Jun 25. Nature. 2014. PMID: 24990740 No abstract available.
-
Cytokines: Tipping TB off balance.Nat Rev Immunol. 2014 Aug;14(8):516. doi: 10.1038/nri3722. Epub 2014 Jul 18. Nat Rev Immunol. 2014. PMID: 25033906 No abstract available.
-
Infectious diseases: tipping TB off balance.Nat Rev Drug Discov. 2014 Aug;13(8):576. doi: 10.1038/nrd4400. Nat Rev Drug Discov. 2014. PMID: 25082284 No abstract available.
Similar articles
-
Targeting the inflammatory response in tuberculosis.N Engl J Med. 2014 Oct 2;371(14):1354-6. doi: 10.1056/NEJMcibr1408663. N Engl J Med. 2014. PMID: 25271609 No abstract available.
-
Cytokines: Tipping TB off balance.Nat Rev Immunol. 2014 Aug;14(8):516. doi: 10.1038/nri3722. Epub 2014 Jul 18. Nat Rev Immunol. 2014. PMID: 25033906 No abstract available.
-
Immunology: Fixing the odds against tuberculosis.Nature. 2014 Jul 3;511(7507):39-40. doi: 10.1038/nature13512. Epub 2014 Jun 25. Nature. 2014. PMID: 24990740 No abstract available.
-
[Development of antituberculous drugs: current status and future prospects].Kekkaku. 2006 Dec;81(12):753-74. Kekkaku. 2006. PMID: 17240921 Review. Japanese.
-
Tuberculosis.In: Holmes KK, Bertozzi S, Bloom BR, Jha P, editors. Major Infectious Diseases. 3rd edition. Washington (DC): The International Bank for Reconstruction and Development / The World Bank; 2017 Nov 3. Chapter 11. In: Holmes KK, Bertozzi S, Bloom BR, Jha P, editors. Major Infectious Diseases. 3rd edition. Washington (DC): The International Bank for Reconstruction and Development / The World Bank; 2017 Nov 3. Chapter 11. PMID: 30212088 Free Books & Documents. Review.
Cited by
-
Differential requirement of formyl peptide receptor 1 in macrophages and neutrophils in the host defense against Mycobacterium tuberculosis Infection.Sci Rep. 2024 Oct 9;14(1):23595. doi: 10.1038/s41598-024-71180-1. Sci Rep. 2024. PMID: 39384825 Free PMC article.
-
Progranulin protects against Clostridioides difficile infection by enhancing IL-22 production.Gut Microbes. 2024 Jan-Dec;16(1):2409220. doi: 10.1080/19490976.2024.2409220. Epub 2024 Sep 30. Gut Microbes. 2024. PMID: 39349385 Free PMC article.
-
Altered hepatic metabolic landscape and insulin sensitivity in response to pulmonary tuberculosis.PLoS Pathog. 2024 Sep 27;20(9):e1012565. doi: 10.1371/journal.ppat.1012565. eCollection 2024 Sep. PLoS Pathog. 2024. PMID: 39331683 Free PMC article.
-
The role of inflammasomes as central inflammatory hubs in Mycobacterium tuberculosis infection.Front Immunol. 2024 Sep 11;15:1436676. doi: 10.3389/fimmu.2024.1436676. eCollection 2024. Front Immunol. 2024. PMID: 39324136 Free PMC article. Review.
References
-
- WHO. Tuberculosis Fact sheet N°104. 2012 http://www.who.int/mediacentre/factsheets/fs104/en/index.html.
-
- Bishai W, Sullivan Z, Bloom BR, Andersen P. Bettering BCG: a tough task for a TB vaccine? Nature Med. 2013;19:410–411. - PubMed
-
- Lawn SD, Zumla AI. Tuberculosis. Lancet. 2011;378:57–72. - PubMed
-
- Gandhi NR, et al. Multidrug-resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosis. Lancet. 2010;375:1830–1843. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

