Proteomic analysis of the multimeric nuclear egress complex of human cytomegalovirus
- PMID: 24969177
- PMCID: PMC4125742
- DOI: 10.1074/mcp.M113.035782
Proteomic analysis of the multimeric nuclear egress complex of human cytomegalovirus
Abstract
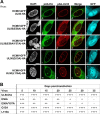
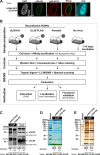
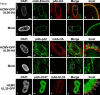
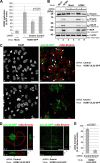
Herpesviral capsids are assembled in the host cell nucleus before being translocated into the cytoplasm for further maturation. The crossing of the nuclear envelope represents a major event that requires the formation of the nuclear egress complex (NEC). Previous studies demonstrated that human cytomegalovirus (HCMV) proteins pUL50 and pUL53, as well as their homologs in all members of Herpesviridae, interact with each other at the nuclear envelope and form the heterodimeric core of the NEC. In order to characterize further the viral and cellular protein content of the multimeric NEC, the native complex was isolated from HCMV-infected human primary fibroblasts at various time points and analyzed using quantitative proteomics. Previously postulated components of the HCMV-specific NEC, as well as novel potential NEC-associated proteins such as emerin, were identified. In this regard, interaction and colocalization between emerin and pUL50 were confirmed by coimmunoprecipitation and confocal microscopy analyses, respectively. A functional validation of viral and cellular NEC constituents was achieved through siRNA-mediated knockdown experiments. The important role of emerin in NEC functionality was demonstrated by a reduction of viral replication when emerin expression was down-regulated. Moreover, under such conditions, reduced production of viral proteins and deregulation of viral late cytoplasmic maturation were observed. Combined, these data prove the functional importance of emerin as an NEC component, associated with pUL50, pUL53, pUL97, p32/gC1qR, and further regulatory proteins. Summarized, our findings provide the first proteomics-based characterization and functional validation of the HCMV-specific multimeric NEC.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


Similar articles
-
Cytomegalovirus pUL50 is the multi-interacting determinant of the core nuclear egress complex (NEC) that recruits cellular accessory NEC components.J Gen Virol. 2016 Jul;97(7):1676-1685. doi: 10.1099/jgv.0.000495. Epub 2016 May 4. J Gen Virol. 2016. PMID: 27145986
-
Properties of Oligomeric Interaction of the Cytomegalovirus Core Nuclear Egress Complex (NEC) and Its Sensitivity to an NEC Inhibitory Small Molecule.Viruses. 2021 Mar 11;13(3):462. doi: 10.3390/v13030462. Viruses. 2021. PMID: 33799898 Free PMC article.
-
The Complex Regulatory Role of Cytomegalovirus Nuclear Egress Protein pUL50 in the Production of Infectious Virus.Cells. 2021 Nov 11;10(11):3119. doi: 10.3390/cells10113119. Cells. 2021. PMID: 34831342 Free PMC article.
-
'Come together'-The Regulatory Interaction of Herpesviral Nuclear Egress Proteins Comprises Both Essential and Accessory Functions.Cells. 2022 Jun 4;11(11):1837. doi: 10.3390/cells11111837. Cells. 2022. PMID: 35681532 Free PMC article. Review.
-
The human cytomegalovirus nuclear egress complex unites multiple functions: Recruitment of effectors, nuclear envelope rearrangement, and docking to nuclear capsids.Rev Med Virol. 2017 Jul;27(4). doi: 10.1002/rmv.1934. Epub 2017 Jun 30. Rev Med Virol. 2017. PMID: 28664574 Review.
Cited by
-
The Interaction between Cyclin B1 and Cytomegalovirus Protein Kinase pUL97 is Determined by an Active Kinase Domain.Viruses. 2015 Aug 11;7(8):4582-601. doi: 10.3390/v7082834. Viruses. 2015. PMID: 26270673 Free PMC article.
-
Role of the Orphan Transporter SLC35E1 in the Nuclear Egress of Herpes Simplex Virus 1.J Virol. 2022 May 25;96(10):e0030622. doi: 10.1128/jvi.00306-22. Epub 2022 Apr 27. J Virol. 2022. PMID: 35475666 Free PMC article.
-
Functional Relevance of the Interaction between Human Cyclins and the Cytomegalovirus-Encoded CDK-Like Protein Kinase pUL97.Viruses. 2021 Jun 27;13(7):1248. doi: 10.3390/v13071248. Viruses. 2021. PMID: 34198986 Free PMC article.
-
The Long Hunt for pssR-Looking for a Phospholipid Synthesis Transcriptional Regulator, Finding the Ribosome.J Bacteriol. 2017 Jun 27;199(14):e00202-17. doi: 10.1128/JB.00202-17. Print 2017 Jul 15. J Bacteriol. 2017. PMID: 28484043 Free PMC article.
-
Proteomic Interaction Patterns between Human Cyclins, the Cyclin-Dependent Kinase Ortholog pUL97 and Additional Cytomegalovirus Proteins.Viruses. 2016 Aug 18;8(8):219. doi: 10.3390/v8080219. Viruses. 2016. PMID: 27548200 Free PMC article.
References
-
- Pe'ery T., Mathews M. B. (2007) Viral Conquest of the Host Cell. In Fields Virology (Knipe D. M., Howley P. M., eds), pp. 169–208, Lippincott Williams & Wilkins, Philadelphia, PA
-
- Mocarski E. S., Shenk T., Pass R. F. (2007) Cytomegaloviruses. In Fields Virology (Knipe D. M., Howley P. M., eds), pp. 2701–2772, Lippincott Williams & Wilkins, Philadelphia, PA
-
- Mettenleiter T. C., Müller F., Granzow H., Klupp B. G. (2013) The way out: what we know and do not know about herpesvirus nuclear egress. Cell. Microbiol. 15, 170–178 - PubMed
-
- Marschall M., Feichtinger S., Milbradt J. (2011) Regulatory roles of protein kinases in cytomegalovirus replication. Adv. Virus Res. 80, 69–101 - PubMed
-
- Johnson D. C., Baines J. D. (2011) Herpesviruses remodel host membranes for virus egress. Nat. Rev. Microbiol. 9, 382–394 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

