CD44-mediated adhesion to hyaluronic acid contributes to mechanosensing and invasive motility
- PMID: 24962319
- PMCID: PMC4201971
- DOI: 10.1158/1541-7786.MCR-13-0629
CD44-mediated adhesion to hyaluronic acid contributes to mechanosensing and invasive motility
Abstract
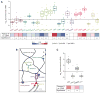
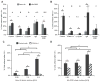
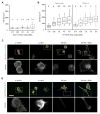
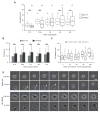
The high-molecular-weight glycosaminoglycan, hyaluronic acid (HA), makes up a significant portion of the brain extracellular matrix. Glioblastoma multiforme (GBM), a highly invasive brain tumor, is associated with aberrant HA secretion, tissue stiffening, and overexpression of the HA receptor CD44. Here, transcriptomic analysis, engineered materials, and measurements of adhesion, migration, and invasion were used to investigate how HA/CD44 ligation contributes to the mechanosensing and invasive motility of GBM tumor cells, both intrinsically and in the context of Arg-Gly-Asp (RGD) peptide/integrin adhesion. Analysis of transcriptomic data from The Cancer Genome Atlas reveals upregulation of transcripts associated with HA/CD44 adhesion. CD44 suppression in culture reduces cell adhesion to HA on short time scales (0.5-hour postincubation) even if RGD is present, whereas maximal adhesion on longer time scales (3 hours) requires both CD44 and integrins. Moreover, time-lapse imaging demonstrates that cell adhesive structures formed during migration on bare HA matrices are more short lived than cellular protrusions formed on surfaces containing RGD. Interestingly, adhesion and migration speed were dependent on HA hydrogel stiffness, implying that CD44-based signaling is intrinsically mechanosensitive. Finally, CD44 expression paired with an HA-rich microenvironment maximized three-dimensional invasion, whereas CD44 suppression or abundant integrin-based adhesion limited it. These findings demonstrate that CD44 transduces HA-based stiffness cues, temporally precedes integrin-based adhesion maturation, and facilitates invasion.
Implications: This study reveals that the CD44 receptor, which is commonly overexpressed in GBM tumors, is critical for cell adhesion, invasion, and mechanosensing of an HA-based matrix.
©2014 American Association for Cancer Research.
Conflict of interest statement
The authors do not disclose any conflicts of interest.
Figures

Similar articles
-
A composite hydrogel platform for the dissection of tumor cell migration at tissue interfaces.Biomaterials. 2014 Oct;35(31):8846-8853. doi: 10.1016/j.biomaterials.2014.07.003. Epub 2014 Jul 19. Biomaterials. 2014. PMID: 25047626 Free PMC article.
-
Glioblastoma Spheroid Invasion through Soft, Brain-Like Matrices Depends on Hyaluronic Acid-CD44 Interactions.Adv Healthc Mater. 2023 Jun;12(14):e2203143. doi: 10.1002/adhm.202203143. Epub 2023 Feb 8. Adv Healthc Mater. 2023. PMID: 36694362 Free PMC article.
-
A mode of cell adhesion and migration facilitated by CD44-dependent microtentacles.Proc Natl Acad Sci U S A. 2020 May 26;117(21):11432-11443. doi: 10.1073/pnas.1914294117. Epub 2020 May 7. Proc Natl Acad Sci U S A. 2020. PMID: 32381732 Free PMC article.
-
Activation of Matrix Hyaluronan-Mediated CD44 Signaling, Epigenetic Regulation and Chemoresistance in Head and Neck Cancer Stem Cells.Int J Mol Sci. 2017 Aug 24;18(9):1849. doi: 10.3390/ijms18091849. Int J Mol Sci. 2017. PMID: 28837080 Free PMC article. Review.
-
CD44 and hyaluronan binding by human myeloid cells.Leuk Lymphoma. 1996 May;21(5-6):407-20, color plates following 528. doi: 10.3109/10428199609093438. Leuk Lymphoma. 1996. PMID: 9172805 Review.
Cited by
-
Residual Disease in Glioma Recurrence: A Dangerous Liaison with Senescence.Cancers (Basel). 2021 Mar 29;13(7):1560. doi: 10.3390/cancers13071560. Cancers (Basel). 2021. PMID: 33805316 Free PMC article. Review.
-
Chondroitin Sulfate and Fucosylated Chondroitin Sulfate as Stimulators of Hematopoiesis in Cyclophosphamide-Induced Mice.Pharmaceuticals (Basel). 2021 Oct 24;14(11):1074. doi: 10.3390/ph14111074. Pharmaceuticals (Basel). 2021. PMID: 34832856 Free PMC article.
-
Hydrogel Arrays Enable Increased Throughput for Screening Effects of Matrix Components and Therapeutics in 3D Tumor Models.J Vis Exp. 2022 Jun 16;(184):10.3791/63791. doi: 10.3791/63791. J Vis Exp. 2022. PMID: 35781280 Free PMC article.
-
Targeting the Tumor Extracellular Matrix by the Natural Molecule 4-Methylumbelliferone: A Complementary and Alternative Cancer Therapeutic Strategy.Front Oncol. 2021 Oct 4;11:710061. doi: 10.3389/fonc.2021.710061. eCollection 2021. Front Oncol. 2021. PMID: 34676159 Free PMC article. Review.
-
Perspective on Translating Biomaterials Into Glioma Therapy: Lessons From in vitro Models.Front Mater. 2018 May;5:27. doi: 10.3389/fmats.2018.00027. Epub 2018 May 9. Front Mater. 2018. PMID: 30911536 Free PMC article.
References
-
- Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–710. - PubMed
-
- Bellail AC, Hunter SB, Brat DJ, Tan C, Van Meir EG. Microregional extracellular matrix heterogeneity in brain modulates glioma cell invasion. Int J Biochem Cell Biol. 2004;36:1046–69. - PubMed
-
- Baier C, Baader SL, Jankowski J, Gieselmann V, Schilling K, Rauch U, et al. Hyaluronan is organized into fiber-like structures along migratory pathways in the developing mouse cerebellum. Matrix Biol. 2007;26:348–58. - PubMed
-
- Bourguignon LYW, Zhu H, Shao L, Chen YW. CD44 interaction with c-Src kinase promotes cortactin-mediated cytoskeleton function and hyaluronic acid-dependent ovarian tumor cell migration. J Biol Chem. 2001;276:7327–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

