The pericyte: a forgotten cell type with important implications for Alzheimer's disease?
- PMID: 24946075
- PMCID: PMC4423607
- DOI: 10.1111/bpa.12152
The pericyte: a forgotten cell type with important implications for Alzheimer's disease?
Abstract
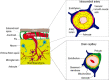
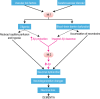
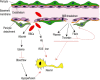
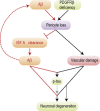
Pericytes are cells in the blood-brain barrier (BBB) that degenerate in Alzheimer's disease (AD), a neurodegenerative disorder characterized by early neurovascular dysfunction, elevation of amyloid β-peptide (Aβ), tau pathology and neuronal loss, leading to progressive cognitive decline and dementia. Pericytes are uniquely positioned within the neurovascular unit between endothelial cells of brain capillaries, astrocytes and neurons. Recent studies have shown that pericytes regulate key neurovascular functions including BBB formation and maintenance, vascular stability and angioarchitecture, regulation of capillary blood flow, and clearance of toxic cellular by-products necessary for normal functioning of the central nervous system (CNS). Here, we review the concept of the neurovascular unit and neurovascular functions of CNS pericytes. Next, we discuss vascular contributions to AD and review new roles of pericytes in the pathogenesis of AD such as vascular-mediated Aβ-independent neurodegeneration, regulation of Aβ clearance and contributions to tau pathology, neuronal loss and cognitive decline. We conclude that future studies should focus on molecular mechanisms and pathways underlying aberrant signal transduction between pericytes and its neighboring cells within the neurovascular unit, that is, endothelial cells, astrocytes and neurons, which could represent potential therapeutic targets to control pericyte degeneration in AD and the resulting secondary vascular and neuronal degeneration.
Keywords: Alzheimer's disease; amyloid beta; blood-brain barrier; hypoperfusion; neurodegeneration; pericytes.
© 2014 International Society of Neuropathology.
Figures

Similar articles
-
Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer's disease.Acta Neuropathol. 2009 Jul;118(1):103-13. doi: 10.1007/s00401-009-0522-3. Epub 2009 Mar 25. Acta Neuropathol. 2009. PMID: 19319544 Free PMC article. Review.
-
Interactions between Beta-Amyloid and Pericytes in Alzheimer's Disease.Front Biosci (Landmark Ed). 2024 Apr 2;29(4):136. doi: 10.31083/j.fbl2904136. Front Biosci (Landmark Ed). 2024. PMID: 38682184 Review.
-
Central nervous system pericytes in health and disease.Nat Neurosci. 2011 Oct 26;14(11):1398-1405. doi: 10.1038/nn.2946. Nat Neurosci. 2011. PMID: 22030551 Free PMC article.
-
Interactions between Amyloid-Β Proteins and Human Brain Pericytes: Implications for the Pathobiology of Alzheimer's Disease.J Clin Med. 2020 May 15;9(5):1490. doi: 10.3390/jcm9051490. J Clin Med. 2020. PMID: 32429102 Free PMC article. Review.
-
Pericyte loss influences Alzheimer-like neurodegeneration in mice.Nat Commun. 2013;4:2932. doi: 10.1038/ncomms3932. Nat Commun. 2013. Retraction in: Nat Commun. 2024 Apr 3;15(1):2882. doi: 10.1038/s41467-024-47285-6 PMID: 24336108 Free PMC article. Retracted.
Cited by
-
Chronic effects of blast injury on the microvasculature in a transgenic mouse model of Alzheimer's disease related Aβ amyloidosis.Fluids Barriers CNS. 2022 Jan 10;19(1):5. doi: 10.1186/s12987-021-00301-z. Fluids Barriers CNS. 2022. PMID: 35012589 Free PMC article.
-
Brain and Retinal Pericytes: Origin, Function and Role.Front Cell Neurosci. 2016 Feb 4;10:20. doi: 10.3389/fncel.2016.00020. eCollection 2016. Front Cell Neurosci. 2016. PMID: 26869887 Free PMC article. Review.
-
Single-cell transcriptome analysis of cavernous tissues reveals the key roles of pericytes in diabetic erectile dysfunction.Elife. 2024 Jun 10;12:RP88942. doi: 10.7554/eLife.88942. Elife. 2024. PMID: 38856719 Free PMC article.
-
Transcriptomic analysis of the 12 major human breast cell types reveals mechanisms of cell and tissue function.PLoS Biol. 2024 Nov 5;22(11):e3002820. doi: 10.1371/journal.pbio.3002820. eCollection 2024 Nov. PLoS Biol. 2024. PMID: 39499736 Free PMC article.
-
Vascular contributions to cognitive impairment/dementia in diabetes: role of endothelial cells and pericytes.Am J Physiol Cell Physiol. 2022 Oct 1;323(4):C1177-C1189. doi: 10.1152/ajpcell.00072.2022. Epub 2022 Aug 29. Am J Physiol Cell Physiol. 2022. PMID: 36036445 Free PMC article. Review.
References
-
- Armulik A, Genove G, Betsholtz C (2011) Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 21:193–215. - PubMed
-
- Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C et al (2010) Pericytes regulate the blood‐brain barrier. Nature 468:557–561. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

