Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling
- PMID: 24930973
- PMCID: PMC4079756
- DOI: 10.1016/j.cmet.2014.05.005
Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling
Abstract
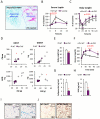
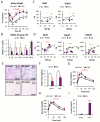
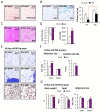
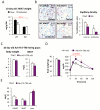
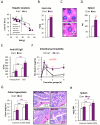
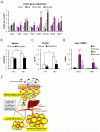
Chronic inflammation constitutes an important link between obesity and its pathophysiological sequelae. In contrast to the belief that inflammatory signals exert a fundamentally negative impact on metabolism, we show that proinflammatory signaling in the adipocyte is in fact required for proper adipose tissue remodeling and expansion. Three mouse models with an adipose tissue-specific reduction in proinflammatory potential were generated that display a reduced capacity for adipogenesis in vivo, while the differentiation potential is unaltered in vitro. Upon high-fat-diet exposure, the expansion of visceral adipose tissue is prominently affected. This is associated with decreased intestinal barrier function, increased hepatic steatosis, and metabolic dysfunction. An impaired local proinflammatory response in the adipocyte leads to increased ectopic lipid accumulation, glucose intolerance, and systemic inflammation. Adipose tissue inflammation is therefore an adaptive response that enables safe storage of excess nutrients and contributes to a visceral depot barrier that effectively filters gut-derived endotoxin.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures

Comment in
-
Obesity: Rethinking inflammation and adipocyte homeostasis.Nat Rev Endocrinol. 2014 Aug;10(8):446. doi: 10.1038/nrendo.2014.103. Epub 2014 Jul 1. Nat Rev Endocrinol. 2014. PMID: 24981459 No abstract available.
Similar articles
-
Anti-adipogenic signals at the onset of obesity-related inflammation in white adipose tissue.Cell Mol Life Sci. 2021 Jan;78(1):227-247. doi: 10.1007/s00018-020-03485-z. Epub 2020 Mar 11. Cell Mol Life Sci. 2021. PMID: 32157317 Free PMC article.
-
Quercetin attenuates adipose hypertrophy, in part through activation of adipogenesis in rats fed a high-fat diet.J Nutr Biochem. 2020 May;79:108352. doi: 10.1016/j.jnutbio.2020.108352. Epub 2020 Feb 4. J Nutr Biochem. 2020. PMID: 32145471
-
Long-term supplementation of honokiol and magnolol ameliorates body fat accumulation, insulin resistance, and adipose inflammation in high-fat fed mice.Mol Nutr Food Res. 2013 Nov;57(11):1988-98. doi: 10.1002/mnfr.201300113. Epub 2013 Jul 31. Mol Nutr Food Res. 2013. PMID: 23901038
-
Evidence for the regulatory role of lipocalin 2 in high-fat diet-induced adipose tissue remodeling in male mice.Endocrinology. 2013 Oct;154(10):3525-38. doi: 10.1210/en.2013-1289. Epub 2013 Jul 24. Endocrinology. 2013. PMID: 23885013 Free PMC article.
-
Control of adipocyte differentiation in different fat depots; implications for pathophysiology or therapy.Front Endocrinol (Lausanne). 2015 Jan 30;6:1. doi: 10.3389/fendo.2015.00001. eCollection 2015. Front Endocrinol (Lausanne). 2015. PMID: 25688231 Free PMC article. Review.
Cited by
-
Adipose tissue plasticity: how fat depots respond differently to pathophysiological cues.Diabetologia. 2016 Jun;59(6):1075-88. doi: 10.1007/s00125-016-3933-4. Epub 2016 Apr 4. Diabetologia. 2016. PMID: 27039901 Free PMC article. Review.
-
Adipocytokines: Are they the Theory of Everything?Cytokine. 2020 Sep;133:155144. doi: 10.1016/j.cyto.2020.155144. Epub 2020 Jun 16. Cytokine. 2020. PMID: 32559663 Free PMC article. Review.
-
A macrophage-collagen fragment axis mediates subcutaneous adipose tissue remodeling in mice.Proc Natl Acad Sci U S A. 2024 Feb 6;121(6):e2313185121. doi: 10.1073/pnas.2313185121. Epub 2024 Feb 1. Proc Natl Acad Sci U S A. 2024. PMID: 38300872 Free PMC article.
-
Hepatic IKKε expression is dispensable for high-fat feeding-induced increases in liver lipid content and alterations in glucose tolerance.Am J Physiol Endocrinol Metab. 2020 Jan 1;318(1):E11-E21. doi: 10.1152/ajpendo.00309.2019. Epub 2019 Oct 29. Am J Physiol Endocrinol Metab. 2020. PMID: 31661298 Free PMC article.
-
Stimulator of Interferon Genes (STING) Triggers Adipocyte Autophagy.Cells. 2023 Sep 24;12(19):2345. doi: 10.3390/cells12192345. Cells. 2023. PMID: 37830559 Free PMC article.
References
-
- Engelman JA, Berg AH, Lewis RY, Lisanti MP, Scherer PE. Tumor necrosis factor alpha-mediated insulin resistance, but not dedifferentiation, is abrogated by MEK1/2 inhibitors in 3T3-L1 adipocytes. Mol Endocrinol. 2000;14:1557–1569. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

