FGF signaling activates a Sox9-Sox10 pathway for the formation and branching morphogenesis of mouse ocular glands
- PMID: 24924191
- PMCID: PMC4067963
- DOI: 10.1242/dev.108944
FGF signaling activates a Sox9-Sox10 pathway for the formation and branching morphogenesis of mouse ocular glands
Abstract
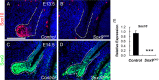
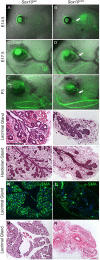
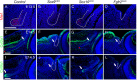
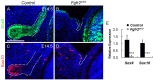
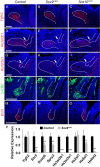
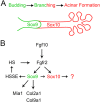
Murine lacrimal, harderian and meibomian glands develop from the prospective conjunctival and eyelid epithelia and produce secretions that lubricate and protect the ocular surface. Sox9 expression localizes to the presumptive conjunctival epithelium as early as E11.5 and is detected in the lacrimal and harderian glands as they form. Conditional deletion showed that Sox9 is required for the development of the lacrimal and harderian glands and contributes to the formation of the meibomian glands. Sox9 regulates the expression of Sox10 to promote the formation of secretory acinar lobes in the lacrimal gland. Sox9 and FGF signaling were required for the expression of cartilage-associated extracellular matrix components during early stage lacrimal gland development. Fgfr2 deletion in the ocular surface epithelium reduced Sox9 and eliminated Sox10 expression. Sox9 deletion from the ectoderm did not affect Fgf10 expression in the adjacent mesenchyme or Fgfr2 expression in the epithelium, but appeared to reduce FGF signaling. Sox9 heterozygotes showed a haploinsufficient phenotype, in which the exorbital branch of the lacrimal gland was absent in most cases. However, enhancement of epithelial FGF signaling by expression of a constitutively active FGF receptor only partially rescued the lacrimal gland defects in Sox9 heterozygotes, suggesting a crucial role of Sox9, downstream of FGF signaling, in regulating lacrimal gland branching and differentiation.
Keywords: FGF signaling; Harderian gland; Lacrimal gland; Sox10; Sox9.
© 2014. Published by The Company of Biologists Ltd.
Figures




Similar articles
-
Barx2 and Fgf10 regulate ocular glands branching morphogenesis by controlling extracellular matrix remodeling.Development. 2011 Aug;138(15):3307-17. doi: 10.1242/dev.066241. Development. 2011. PMID: 21750040 Free PMC article.
-
Molecular regulation of ocular gland development.Semin Cell Dev Biol. 2019 Jul;91:66-74. doi: 10.1016/j.semcdb.2018.07.023. Epub 2018 Sep 25. Semin Cell Dev Biol. 2019. PMID: 30266427 Review.
-
FGF10 is an inducer and Pax6 a competence factor for lacrimal gland development.Development. 2000 Jun;127(12):2563-72. doi: 10.1242/dev.127.12.2563. Development. 2000. PMID: 10821755
-
Fgf10 and Sox9 are essential for the establishment of distal progenitor cells during mouse salivary gland development.Development. 2017 Jun 15;144(12):2294-2305. doi: 10.1242/dev.146019. Epub 2017 May 15. Development. 2017. PMID: 28506998 Free PMC article.
-
Major ocular glands (harderian gland and lacrimal gland) of the musk shrew (Suncus murinus) with a review on the comparative anatomy and histology of the mammalian lacrimal glands.J Morphol. 1989 Jul;201(1):39-57. doi: 10.1002/jmor.1052010105. J Morphol. 1989. PMID: 2664187 Review.
Cited by
-
Salivary gland stem cells: A review of development, regeneration and cancer.Genesis. 2018 May;56(5):e23211. doi: 10.1002/dvg.23211. Epub 2018 May 4. Genesis. 2018. PMID: 29663717 Free PMC article. Review.
-
Lacrimal Gland Repair Using Progenitor Cells.Stem Cells Transl Med. 2017 Jan;6(1):88-98. doi: 10.5966/sctm.2016-0191. Epub 2016 Aug 15. Stem Cells Transl Med. 2017. PMID: 28170196 Free PMC article.
-
Lacrimal gland development: From signaling interactions to regenerative medicine.Dev Dyn. 2017 Dec;246(12):970-980. doi: 10.1002/dvdy.24551. Epub 2017 Aug 18. Dev Dyn. 2017. PMID: 28710815 Free PMC article. Review.
-
Alteration in cellular turnover and progenitor cell population in lacrimal glands from thrombospondin 1-/- mice, a model of dry eye.Exp Eye Res. 2016 Dec;153:27-41. doi: 10.1016/j.exer.2016.09.011. Epub 2016 Sep 30. Exp Eye Res. 2016. PMID: 27697548 Free PMC article.
-
A closer look into the cellular and molecular biology of myoepithelial cells across various exocrine glands.Ocul Surf. 2024 Jan;31:63-80. doi: 10.1016/j.jtos.2023.12.003. Epub 2023 Dec 21. Ocul Surf. 2024. PMID: 38141817 Free PMC article. Review.
References
-
- Akiyama H., Chaboissier M.-C., Martin J. F., Schedl A., de Crombrugghe B. (2002). The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 16, 2813-2828 10.1101/gad.1017802 - DOI - PMC - PubMed
-
- Belteki G., Haigh J., Kabacs N., Haigh K., Sison K., Costantini F., Whitsett J., Quaggin S. E., Nagy A. (2005). Conditional and inducible transgene expression in mice through the combinatorial use of Cre-mediated recombination and tetracycline induction. Nucleic Acids Res. 33, e51 10.1093/nar/gni051 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

