Astrocyte-derived BDNF supports myelin protein synthesis after cuprizone-induced demyelination
- PMID: 24920623
- PMCID: PMC4051974
- DOI: 10.1523/JNEUROSCI.4267-13.2014
Astrocyte-derived BDNF supports myelin protein synthesis after cuprizone-induced demyelination
Abstract
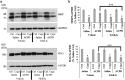
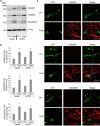
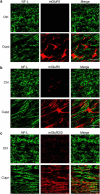
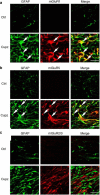
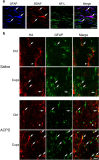
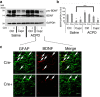
It is well established that BDNF may enhance oligodendrocyte differentiation following a demyelinating lesion, however, the endogenous sources of BDNF that may be harnessed to reverse deficits associated with such lesions are poorly defined. Here, we investigate roles of astrocytes in synthesizing and releasing BDNF. These cells are known to express BDNF following injury in vivo. In culture, they increase BDNF synthesis and release in response to glutamate metabotropic stimulation. Following cuprizone-elicited demyelination in mice, astrocytes contain BDNF and increase levels of metabotropic receptors. The metabotropic agonist, trans-(1S,3R)-1-amino-1,3-cyclopentanedicarboxylic acid (ACPD), was therefore injected into the demyelinating lesion. Increases in BDNF, as well as myelin proteins, were observed. Effects of ACPD were eliminated by coinjection of trkB-Fc to locally deplete BDNF and by deletion of astrocyte-derived BDNF. The data indicate that astrocyte-derived BDNF may be a source of trophic support that can be used to reverse deficits elicited following demyelination.
Keywords: ACPD; BDNF; astrocytes; cuprizone; metabotropic receptors; oligodendrocytes.
Copyright © 2014 the authors 0270-6474/14/348186-11$15.00/0.
Figures


Similar articles
-
CHPG enhances BDNF and myelination in cuprizone-treated mice through astrocytic metabotropic glutamate receptor 5.Glia. 2021 Aug;69(8):1950-1965. doi: 10.1002/glia.24003. Epub 2021 Apr 3. Glia. 2021. PMID: 33811383 Free PMC article.
-
Brain-derived neurotrophic factor deficiency restricts proliferation of oligodendrocyte progenitors following cuprizone-induced demyelination.ASN Neuro. 2015 Jan 13;7(1):1759091414566878. doi: 10.1177/1759091414566878. Print 2015 Jan-Feb. ASN Neuro. 2015. PMID: 25586993 Free PMC article.
-
Reduced cuprizone-induced cerebellar demyelination in mice with astrocyte-targeted production of IL-6 is associated with chronically activated, but less responsive microglia.J Neuroimmunol. 2017 Sep 15;310:97-102. doi: 10.1016/j.jneuroim.2017.07.003. Epub 2017 Jul 6. J Neuroimmunol. 2017. PMID: 28778453
-
Astrocytes Promote Oligodendrogenesis after White Matter Damage via Brain-Derived Neurotrophic Factor.J Neurosci. 2015 Oct 14;35(41):14002-8. doi: 10.1523/JNEUROSCI.1592-15.2015. J Neurosci. 2015. PMID: 26468200 Free PMC article.
-
Targeting TrkB with a Brain-Derived Neurotrophic Factor Mimetic Promotes Myelin Repair in the Brain.J Neurosci. 2018 Aug 8;38(32):7088-7099. doi: 10.1523/JNEUROSCI.0487-18.2018. Epub 2018 Jul 5. J Neurosci. 2018. PMID: 29976621 Free PMC article.
Cited by
-
Roles of astrocytes in response to aging, Alzheimer's disease and multiple sclerosis.Brain Res. 2021 Aug 1;1764:147464. doi: 10.1016/j.brainres.2021.147464. Epub 2021 Apr 1. Brain Res. 2021. PMID: 33812850 Free PMC article. Review.
-
Ultrasound Neuromodulation Reduces Demyelination in a Rat Model of Multiple Sclerosis.Int J Mol Sci. 2022 Sep 2;23(17):10034. doi: 10.3390/ijms231710034. Int J Mol Sci. 2022. PMID: 36077437 Free PMC article.
-
Reactive Transformation and Increased BDNF Signaling by Hippocampal Astrocytes in Response to MK-801.PLoS One. 2015 Dec 23;10(12):e0145651. doi: 10.1371/journal.pone.0145651. eCollection 2015. PLoS One. 2015. PMID: 26700309 Free PMC article.
-
Phenytoin promotes the proliferation of oligodendrocytes and enhances the expression of myelin basic protein in the corpus callosum of mice demyelinated by cuprizone.Exp Brain Res. 2022 May;240(5):1617-1627. doi: 10.1007/s00221-022-06356-0. Epub 2022 Apr 1. Exp Brain Res. 2022. PMID: 35362723
-
White Matter Tracts and Diffuse Lower-Grade Gliomas: The Pivotal Role of Myelin Plasticity in the Tumor Pathogenesis, Infiltration Patterns, Functional Consequences and Therapeutic Management.Front Oncol. 2022 Mar 2;12:855587. doi: 10.3389/fonc.2022.855587. eCollection 2022. Front Oncol. 2022. PMID: 35311104 Free PMC article. Review.
References
-
- Agari T, Yasuhara T, Matsui T, Kuramoto S, Kondo A, Miyoshi Y, Shingo T, Borlongan CV, Date I. Intrapallidal metabotropic glutamate receptor activation in a rat model of Parkinson's disease: behavioral and histological analyses. Brain Res. 2008;1203:189–196. doi: 10.1016/j.brainres.2008.01.051. - DOI - PubMed
-
- Aharoni R, Eilam R, Domev H, Labunskay G, Sela M, Arnon R. The immunomodulator glatiramer acetate augments the expression of neurotrophic factors in brains of experimental autoimmune encephalomyelitis mice. Proc Natl Acad Sci U S A. 2005;102:19045–19050. doi: 10.1073/pnas.0509438102. - DOI - PMC - PubMed
-
- Aronica E, Catania MV, Geurts J, Yankaya B, Troost D. Immunohistochemical localization of group I and II metabotropic glutamate receptors in control and amyotrophic lateral sclerosis human spinal cord: upregulation in reactive astrocytes. Neuroscience. 2001;105:509–520. doi: 10.1016/S0306-4522(01)00181-6. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
