Oxytocin is an age-specific circulating hormone that is necessary for muscle maintenance and regeneration
- PMID: 24915299
- PMCID: PMC4512838
- DOI: 10.1038/ncomms5082
Oxytocin is an age-specific circulating hormone that is necessary for muscle maintenance and regeneration
Abstract
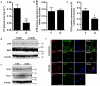
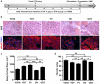
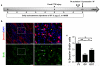
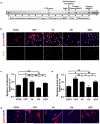
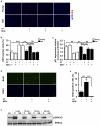
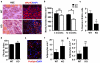
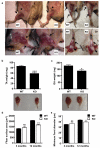
The regenerative capacity of skeletal muscle declines with age. Previous studies suggest that this process can be reversed by exposure to young circulation; however, systemic age-specific factors responsible for this phenomenon are largely unknown. Here we report that oxytocin--a hormone best known for its role in lactation, parturition and social behaviours--is required for proper muscle tissue regeneration and homeostasis, and that plasma levels of oxytocin decline with age. Inhibition of oxytocin signalling in young animals reduces muscle regeneration, whereas systemic administration of oxytocin rapidly improves muscle regeneration by enhancing aged muscle stem cell activation/proliferation through activation of the MAPK/ERK signalling pathway. We further show that the genetic lack of oxytocin does not cause a developmental defect in muscle but instead leads to premature sarcopenia. Considering that oxytocin is an FDA-approved drug, this work reveals a potential novel and safe way to combat or prevent skeletal muscle ageing.
Figures
Similar articles
-
Thyroid Hormone Receptor Alpha is Essential to Maintain the Satellite Cell Niche During Skeletal Muscle Injury and Sarcopenia of Aging.Thyroid. 2017 Oct;27(10):1316-1322. doi: 10.1089/thy.2017.0021. Thyroid. 2017. PMID: 28847239 Free PMC article.
-
Prolonged absence of myostatin reduces sarcopenia.J Cell Physiol. 2006 Dec;209(3):866-73. doi: 10.1002/jcp.20778. J Cell Physiol. 2006. PMID: 16972257
-
Skeletal muscle stem cells: effects of aging and metabolism on muscle regenerative function.Cold Spring Harb Symp Quant Biol. 2011;76:101-11. doi: 10.1101/sqb.2011.76.010652. Epub 2011 Sep 29. Cold Spring Harb Symp Quant Biol. 2011. PMID: 21960527 Review.
-
Functional dysregulation of stem cells during aging: a focus on skeletal muscle stem cells.FEBS J. 2013 Sep;280(17):4051-62. doi: 10.1111/febs.12221. Epub 2013 Mar 21. FEBS J. 2013. PMID: 23452120 Review.
-
Therapies for sarcopenia and regeneration of old skeletal muscles: more a case of old tissue architecture than old stem cells.Bioarchitecture. 2014;4(3):81-7. doi: 10.4161/bioa.29668. Epub 2014 Jul 28. Bioarchitecture. 2014. PMID: 25101758 Free PMC article. Review.
Cited by
-
Severely Damaged Freeze-Injured Skeletal Muscle Reveals Functional Impairment, Inadequate Repair, and Opportunity for Human Stem Cell Application.Biomedicines. 2023 Dec 21;12(1):30. doi: 10.3390/biomedicines12010030. Biomedicines. 2023. PMID: 38275391 Free PMC article.
-
Integrating Genome-Wide Association Study with RNA-Sequencing Reveals HDAC9 as a Candidate GeneInfluencing Loin Muscle Area in Beijing Black Pigs.Biology (Basel). 2022 Nov 8;11(11):1635. doi: 10.3390/biology11111635. Biology (Basel). 2022. PMID: 36358336 Free PMC article.
-
Oxytocin and elderly MRI-based hippocampus and amygdala volume: a 7-year follow-up study.Brain Commun. 2020 Jun 11;2(2):fcaa081. doi: 10.1093/braincomms/fcaa081. eCollection 2020. Brain Commun. 2020. PMID: 32954331 Free PMC article.
-
Identification of oxytocin receptor activating chemical components from traditional Japanese medicines.J Food Drug Anal. 2021 Dec 15;29(4):653-675. doi: 10.38212/2224-6614.3381. J Food Drug Anal. 2021. PMID: 35649140 Free PMC article.
-
Age-related dysfunction of the DNA damage response in intestinal stem cells.Inflamm Regen. 2019 Apr 26;39:8. doi: 10.1186/s41232-019-0096-y. eCollection 2019. Inflamm Regen. 2019. PMID: 31057688 Free PMC article.
References
-
- Berger MJ, Doherty TJ. Sarcopenia: prevalence, mechanisms, and functional consequences. Interdiscip Top Gerontol. 2010;37:94–114. - PubMed
-
- Conboy IM, et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. - PubMed
-
- Brack AS, et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

