An unexpected twist to the activation of IKKβ: TAK1 primes IKKβ for activation by autophosphorylation
- PMID: 24911653
- PMCID: PMC4206954
- DOI: 10.1042/BJ20140444
An unexpected twist to the activation of IKKβ: TAK1 primes IKKβ for activation by autophosphorylation
Abstract
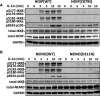
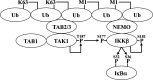
IKKβ {IκB [inhibitor of NF-κB (nuclear factor κB)] kinase β} is required to activate the transcription factor NF-κB, but how IKKβ itself is activated in vivo is still unclear. It was found to require phosphorylation by one or more 'upstream' protein kinases in some reports, but by autophosphorylation in others. In the present study, we resolve this contro-versy by demonstrating that the activation of IKKβ induced by IL-1 (interleukin-1) or TNF (tumour necrosis factor) in embryonic fibroblasts, or by ligands that activate Toll-like receptors in macrophages, requires two distinct phosphorylation events: first, the TAK1 [TGFβ (transforming growth factor β)-activated kinase-1]-catalysed phosphorylation of Ser¹⁷⁷ and, secondly, the IKKβ-catalysed autophosphorylation of Ser¹⁸¹. The phosphorylation of Ser¹⁷⁷ by TAK1 is a priming event required for the subsequent autophosphorylation of Ser¹⁸¹, which enables IKKβ to phosphorylate exogenous substrates. We also provide genetic evidence which indicates that the IL-1-stimulated, LUBAC (linear ubiquitin chain assembly complex)-catalysed formation of linear ubiquitin chains and their interaction with the NEMO (NF-κB essential modulator) component of the canonical IKK complex permits the TAK1-catalysed priming phosphorylation of IKKβ at Ser¹⁷⁷ and IKKα at Ser¹⁷⁶. These findings may be of general significance for the activation of other protein kinases.
Figures



Comment in
-
Priming IKKβ kinase for action.Biochem J. 2014 Oct 1;463(1):e1-2. doi: 10.1042/BJ20140989. Biochem J. 2014. PMID: 25195736
Similar articles
-
Kaposi's sarcoma associated herpesvirus encoded viral FLICE inhibitory protein K13 activates NF-κB pathway independent of TRAF6, TAK1 and LUBAC.PLoS One. 2012;7(5):e36601. doi: 10.1371/journal.pone.0036601. Epub 2012 May 8. PLoS One. 2012. PMID: 22590573 Free PMC article.
-
Priming IKKβ kinase for action.Biochem J. 2014 Oct 1;463(1):e1-2. doi: 10.1042/BJ20140989. Biochem J. 2014. PMID: 25195736
-
Protein Kinase-Mediated Decision Between the Life and Death.Adv Exp Med Biol. 2021;1275:1-33. doi: 10.1007/978-3-030-49844-3_1. Adv Exp Med Biol. 2021. PMID: 33539010
-
Regulation and function of IKK and IKK-related kinases.Sci STKE. 2006 Oct 17;2006(357):re13. doi: 10.1126/stke.3572006re13. Sci STKE. 2006. PMID: 17047224 Review.
-
The role of hybrid ubiquitin chains in the MyD88 and other innate immune signalling pathways.Cell Death Differ. 2017 Jul;24(7):1153-1159. doi: 10.1038/cdd.2017.17. Epub 2017 May 5. Cell Death Differ. 2017. PMID: 28475177 Free PMC article. Review.
Cited by
-
Systems Biology Methods via Genome-Wide RNA Sequences to Investigate Pathogenic Mechanisms for Identifying Biomarkers and Constructing a DNN-Based Drug-Target Interaction Model to Predict Potential Molecular Drugs for Treating Atopic Dermatitis.Int J Mol Sci. 2024 Oct 4;25(19):10691. doi: 10.3390/ijms251910691. Int J Mol Sci. 2024. PMID: 39409019 Free PMC article.
-
Ubiquitin in the activation and attenuation of innate antiviral immunity.J Exp Med. 2016 Jan 11;213(1):1-13. doi: 10.1084/jem.20151531. Epub 2015 Dec 28. J Exp Med. 2016. PMID: 26712804 Free PMC article. Review.
-
NFκB signalling in colorectal cancer: Examining the central dogma of IKKα and IKKβ signalling.Heliyon. 2024 Jun 12;10(12):e32904. doi: 10.1016/j.heliyon.2024.e32904. eCollection 2024 Jun 30. Heliyon. 2024. PMID: 38975078 Free PMC article. Review.
-
Regulatory subunit NEMO promotes polyubiquitin-dependent induction of NF-κB through a targetable second interaction with upstream activator IKK2.J Biol Chem. 2022 May;298(5):101864. doi: 10.1016/j.jbc.2022.101864. Epub 2022 Mar 24. J Biol Chem. 2022. PMID: 35339487 Free PMC article.
-
A central role of IKK2 and TPL2 in JNK activation and viral B-cell transformation.Nat Commun. 2020 Feb 4;11(1):685. doi: 10.1038/s41467-020-14502-x. Nat Commun. 2020. PMID: 32019925 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

