Drug resistance to targeted therapies: déjà vu all over again
- PMID: 24910388
- PMCID: PMC5528618
- DOI: 10.1016/j.molonc.2014.05.004
Drug resistance to targeted therapies: déjà vu all over again
Abstract
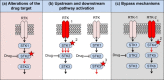
A major limitation of targeted anticancer therapies is intrinsic or acquired resistance. This review emphasizes similarities in the mechanisms of resistance to endocrine therapies in breast cancer and those seen with the new generation of targeted cancer therapeutics. Resistance to single-agent cancer therapeutics is frequently the result of reactivation of the signaling pathway, indicating that a major limitation of targeted agents lies in their inability to fully block the cancer-relevant signaling pathway. The development of mechanism-based combinations of targeted therapies together with non-invasive molecular disease monitoring is a logical way forward to delay and ultimately overcome drug resistance development.
Keywords: Anticancer therapy; Drug combinations; Drug resistance; Endocrine therapy; Pathway reactivation; Targeted therapy.
Copyright © 2014 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures

Similar articles
-
Mapping the Pathways of Resistance to Targeted Therapies.Cancer Res. 2015 Oct 15;75(20):4247-51. doi: 10.1158/0008-5472.CAN-15-1248. Epub 2015 Sep 21. Cancer Res. 2015. PMID: 26392071 Free PMC article. Review.
-
Mechanisms of acquired resistance to targeted cancer therapies.Future Oncol. 2012 Aug;8(8):999-1014. doi: 10.2217/fon.12.86. Future Oncol. 2012. PMID: 22894672 Review.
-
Nanomedicine for targeted cancer therapy: towards the overcoming of drug resistance.Drug Resist Updat. 2011 Jun;14(3):150-63. doi: 10.1016/j.drup.2011.01.003. Epub 2011 Feb 16. Drug Resist Updat. 2011. PMID: 21330184 Review.
-
The roadmap of TRAIL apoptotic pathway-targeted cancer therapies: What is next?Expert Rev Anticancer Ther. 2012 May;12(5):547-9. doi: 10.1586/era.12.33. Expert Rev Anticancer Ther. 2012. PMID: 22594889 No abstract available.
-
Highlights in Resistance Mechanism Pathways for Combination Therapy.Cells. 2019 Aug 30;8(9):1013. doi: 10.3390/cells8091013. Cells. 2019. PMID: 31480389 Free PMC article. Review.
Cited by
-
Mutational interactions define novel cancer subgroups.Nat Commun. 2018 Oct 19;9(1):4353. doi: 10.1038/s41467-018-06867-x. Nat Commun. 2018. PMID: 30341300 Free PMC article.
-
The combination of the glycolysis inhibitor 2-DG and sorafenib can be effective against sorafenib-tolerant persister cancer cells.Onco Targets Ther. 2019 Jul 8;12:5359-5373. doi: 10.2147/OTT.S212465. eCollection 2019. Onco Targets Ther. 2019. PMID: 31371980 Free PMC article.
-
Genetic screens reveal new targetable vulnerabilities in BAP1-deficient mesothelioma.Cell Rep Med. 2023 Feb 21;4(2):100915. doi: 10.1016/j.xcrm.2022.100915. Epub 2023 Jan 18. Cell Rep Med. 2023. PMID: 36657447 Free PMC article.
-
Ferroptosis and Its Multifaceted Role in Cancer: Mechanisms and Therapeutic Approach.Antioxidants (Basel). 2022 Jul 31;11(8):1504. doi: 10.3390/antiox11081504. Antioxidants (Basel). 2022. PMID: 36009223 Free PMC article. Review.
-
Critical role of HMGA proteins in cancer cell chemoresistance.J Mol Med (Berl). 2017 Apr;95(4):353-360. doi: 10.1007/s00109-017-1520-x. Epub 2017 Mar 14. J Mol Med (Berl). 2017. PMID: 28293697 Review.
References
-
- Albertson, D.G. , 2012. ESR1 amplification in breast cancer: controversy resolved?. J. Pathol. 227, 1–3. - PubMed
-
- Amado, R.G. , Wolf, M. , Peeters, M. , Van Cutsem, E. , Siena, S. , Freeman, D.J. , Juan, T. , Sikorski, R. , Suggs, S. , Radinsky, R. , Patterson, S.D. , Chang, D.D. , 2008. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J. Clin. Oncol. 26, 1626–1634. - PubMed
-
- Arpino, G. , Green, S.J. , Allred, D.C. , Lew, D. , Martino, S. , Osborne, C.K. , Elledge, R.M. , 2004. HER-2 amplification, HER-1 expression, and tamoxifen response in estrogen receptor-positive metastatic breast cancer: a southwest oncology group study. Clin. Cancer Res. 10, 5670–5676. - PubMed
-
- Awad, M.M. , Katayama, R. , McTigue, M. , Liu, W. , Deng, Y.L. , Brooun, A. , Friboulet, L. , Huang, D. , Falk, M.D. , Timofeevski, S. , Wilner, K.D. , Lockerman, E.L. , Khan, T.M. , Mahmood, S. , Gainor, J.F. , Digumarthy, S.R. , Stone, J.R. , Mino-Kenudson, M. , Christensen, J.G. , Iafrate, A.J. , Engelman, J.A. , Shaw, A.T. , 2013. Acquired resistance to crizotinib from a mutation in CD74-ROS1. N. Engl. J. Med. 368, 2395–2401. - PMC - PubMed
-
- Bachelot, T. , Bourgier, C. , Cropet, C. , Ray-Coquard, I. , Ferrero, J.M. , Freyer, G. , Abadie-Lacourtoisie, S. , Eymard, J.C. , Debled, M. , Spaeth, D. , Legouffe, E. , Allouache, D. , El Kouri, C. , Pujade-Lauraine, E. , 2012. Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO study. J. Clin. Oncol. 30, 2718–2724. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

