TRAIL-R2-specific antibodies and recombinant TRAIL can synergise to kill cancer cells
- PMID: 24909167
- PMCID: PMC4240732
- DOI: 10.1038/onc.2014.156
TRAIL-R2-specific antibodies and recombinant TRAIL can synergise to kill cancer cells
Abstract
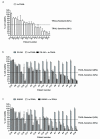
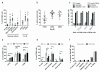
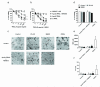
Tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) induces apoptosis in cancer cells while sparing normal tissues. Despite promising preclinical results, few patients responded to treatment with recombinant TRAIL (Apo2L/Dulanermin) or TRAIL-R2-specific antibodies, such as conatumumab (AMG655). It is unknown whether this was due to intrinsic TRAIL resistance within primary human cancers or insufficient agonistic activity of the TRAIL-receptor (TRAIL-R)-targeting drugs. Fcγ receptors (FcγR)-mediated crosslinking increases the cancer-cell-killing activity of TRAIL-R2-specific antibodies in vivo. We tested this phenomenon using FcγR-expressing immune cells from patients with ovarian cancer. However, even in the presence of high numbers of FcγR-expressing immune cells, as found in ovarian cancer ascites, AMG655-induced apoptosis was not enabled to any significant degree, indicating that this concept may not translate into clinical use. On the basis of these results, we next set out to determine whether AMG655 possibly interferes with apoptosis induction by endogenous TRAIL, which could be expressed by immune cells. To do so, we tested how AMG655 affected apoptosis induction by recombinant TRAIL. This, however, resulted in the surprising discovery of a striking synergy between AMG655 and non-tagged TRAIL (Apo2L/TRAIL) in killing cancer cells. This combination was as effective in killing cancer cells as highly active recombinant isoleucine-zipper-tagged TRAIL (iz-TRAIL). The increased killing efficiency was due to enhanced formation of the TRAIL death-inducing signalling complex, enabled by concomitant binding of Apo2L/TRAIL and AMG655 to TRAIL-R2. The synergy of AMG655 with Apo2L/TRAIL extended to primary ovarian cancer cells and was further enhanced by combination with the proteasome inhibitor bortezomib or a second mitochondrial-derived activator of caspases (SMAC) mimetic. Importantly, primary human hepatocytes were not killed by the AMG655-Apo2L/TRAIL combination, also not when further combined with bortezomib or a SMAC mimetic. We therefore propose that clinical-grade non-tagged recombinant forms of TRAIL, such as dulanermin, could be combined with antibodies such as AMG655 to introduce a highly active TRAIL-R2-agonistic therapy into the cancer clinic.
Figures
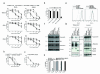
Similar articles
-
Activity of selective fully human agonistic antibodies to the TRAIL death receptors TRAIL-R1 and TRAIL-R2 in primary and cultured lymphoma cells: induction of apoptosis and enhancement of doxorubicin- and bortezomib-induced cell death.Br J Haematol. 2005 Aug;130(4):501-10. doi: 10.1111/j.1365-2141.2005.05656.x. Br J Haematol. 2005. PMID: 16098063
-
Bortezomib sensitizes non-Hodgkin's lymphoma cells to apoptosis induced by antibodies to tumor necrosis factor related apoptosis-inducing ligand (TRAIL) receptors TRAIL-R1 and TRAIL-R2.Clin Cancer Res. 2007 Sep 15;13(18 Pt 2):5528s-5534s. doi: 10.1158/1078-0432.CCR-07-0982. Clin Cancer Res. 2007. PMID: 17875785
-
Mapatumumab and lexatumumab induce apoptosis in TRAIL-R1 and TRAIL-R2 antibody-resistant NSCLC cell lines when treated in combination with bortezomib.Mol Cancer Ther. 2009 Feb;8(2):292-302. doi: 10.1158/1535-7163.MCT-08-0918. Epub 2009 Jan 27. Mol Cancer Ther. 2009. PMID: 19174554
-
Combining proteasome inhibition with TNF-related apoptosis-inducing ligand (Apo2L/TRAIL) for cancer therapy.Cancer Immunol Immunother. 2006 Jan;55(1):76-84. doi: 10.1007/s00262-005-0676-3. Epub 2005 Oct 27. Cancer Immunol Immunother. 2006. PMID: 15864587 Free PMC article. Review.
-
TRAIL receptor signalling and modulation: Are we on the right TRAIL?Cancer Treat Rev. 2009 May;35(3):280-8. doi: 10.1016/j.ctrv.2008.11.006. Epub 2008 Dec 30. Cancer Treat Rev. 2009. PMID: 19117685 Review.
Cited by
-
CD19 chimeric antigen receptor (CD19 CAR)-redirected adoptive T-cell immunotherapy for the treatment of relapsed or refractory B-cell Non-Hodgkin's Lymphomas.Am J Cancer Res. 2016 Jan 15;6(2):403-24. eCollection 2016. Am J Cancer Res. 2016. PMID: 27186412 Free PMC article. Review.
-
Importance of TRAIL Molecular Anatomy in Receptor Oligomerization and Signaling. Implications for Cancer Therapy.Cancers (Basel). 2019 Mar 29;11(4):444. doi: 10.3390/cancers11040444. Cancers (Basel). 2019. PMID: 30934872 Free PMC article. Review.
-
Designed proteins assemble antibodies into modular nanocages.Science. 2021 Apr 2;372(6537):eabd9994. doi: 10.1126/science.abd9994. Science. 2021. PMID: 33795432 Free PMC article.
-
Generation and characterization of novel anti-DR4 and anti-DR5 antibodies developed by genetic immunization.Cell Death Dis. 2019 Feb 4;10(2):101. doi: 10.1038/s41419-019-1343-5. Cell Death Dis. 2019. PMID: 30718507 Free PMC article.
-
Designed proteins assemble antibodies into modular nanocages.bioRxiv [Preprint]. 2020 Dec 1:2020.12.01.406611. doi: 10.1101/2020.12.01.406611. bioRxiv. 2020. Update in: Science. 2021 Apr 2;372(6537):eabd9994. doi: 10.1126/science.abd9994. PMID: 33299994 Free PMC article. Updated. Preprint.
References
-
- Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5(2):157–63. Epub 1999/02/04. - PubMed
-
- Lawrence D, Shahrokh Z, Marsters S, Achilles K, Shih D, Mounho B, et al. Differential hepatocyte toxicity of recombinant Apo2L/TRAIL versions. Nat Med. United States. 2001:383–5. - PubMed
-
- Ganten TM, Koschny R, Sykora J, Schulze-Bergkamen H, Buchler P, Haas TL, et al. Preclinical differentiation between apparently safe and potentially hepatotoxic applications of TRAIL either alone or in combination with chemotherapeutic drugs. Clinical cancer research: an official journal of the American Association for Cancer Research. 2006;12(8):2640–6. Epub 2006/04/28. - PubMed
-
- Herbst RS, Eckhardt SG, Kurzrock R, Ebbinghaus S, O’Dwyer PJ, Gordon MS, et al. Phase I dose-escalation study of recombinant human Apo2L/TRAIL, a dual proapoptotic receptor agonist, in patients with advanced cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28(17):2839–46. Epub 2010/05/12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

