Neuroprotective Tri- and Tetracyclic BChE Inhibitors Releasing Reversible Inhibitors upon Carbamate Transfer
- PMID: 24900407
- PMCID: PMC4025739
- DOI: 10.1021/ml3001825
Neuroprotective Tri- and Tetracyclic BChE Inhibitors Releasing Reversible Inhibitors upon Carbamate Transfer
Abstract
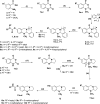
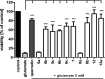
Tri- and tetracyclic nitrogen-bridgehead compounds were designed and synthesized to yield micromolar cholinesterase (ChE) inhibitors. Structure-activity relationships identified potent compounds with butyrylcholinesterase selectivity. These compounds were selected as starting points for the design and synthesis of carbamate-based (pseudo)irreversible inhibitors. Compounds with superior inhibitory activity and selectivity were obtained and kinetically characterized also with regard to the velocity of enzyme carbamoylation. Structural elements were identified and introduced that additionally showed neuroprotective properties on a hippocampal neuronal cell line (HT-22) after glutamate-induced intracellular reactive oxygen species generation. We have identified potent and selective pseudoirreversible butyrylcholinesterase inhibitors that release reversible inhibitors with neuroprotective properties after carbamate transfer to the active site of cholinesterases.
Keywords: Alzheimer's disease; butyrylcholinesterase; carbamates; neuroprotection.
Figures
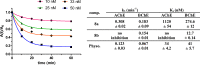
Similar articles
-
Cyclic acyl guanidines bearing carbamate moieties allow potent and dirigible cholinesterase inhibition of either acetyl- or butyrylcholinesterase.Bioorg Med Chem. 2014 Sep 1;22(17):5020-34. doi: 10.1016/j.bmc.2014.06.010. Epub 2014 Jun 26. Bioorg Med Chem. 2014. PMID: 25059502
-
Highly Selective Butyrylcholinesterase Inhibitors with Tunable Duration of Action by Chemical Modification of Transferable Carbamate Units Exhibit Pronounced Neuroprotective Effect in an Alzheimer's Disease Mouse Model.J Med Chem. 2019 Oct 24;62(20):9116-9140. doi: 10.1021/acs.jmedchem.9b01012. Epub 2019 Oct 14. J Med Chem. 2019. PMID: 31609115
-
N-alkylpiperidine carbamates as potential anti-Alzheimer's agents.Eur J Med Chem. 2020 Jul 1;197:112282. doi: 10.1016/j.ejmech.2020.112282. Epub 2020 Apr 15. Eur J Med Chem. 2020. PMID: 32380361
-
Discovery of Highly Selective and Nanomolar Carbamate-Based Butyrylcholinesterase Inhibitors by Rational Investigation into Their Inhibition Mode.J Med Chem. 2016 Mar 10;59(5):2067-82. doi: 10.1021/acs.jmedchem.5b01674. Epub 2016 Feb 17. J Med Chem. 2016. PMID: 26886849
-
Cholinesterase Inhibitory Activity of Some semi-Rigid Spiro Heterocycles: POM Analyses and Crystalline Structure of Pharmacophore Site.Mini Rev Med Chem. 2018;18(8):711-716. doi: 10.2174/1389557517666170713114039. Mini Rev Med Chem. 2018. PMID: 28714400 Review.
Cited by
-
Experimental and theoretical investigations into the stability of cyclic aminals.Beilstein J Org Chem. 2016 Oct 31;12:2280-2292. doi: 10.3762/bjoc.12.221. eCollection 2016. Beilstein J Org Chem. 2016. PMID: 28144295 Free PMC article.
-
From tryptamine to the discovery of efficient multi-target directed ligands against cholinesterase-associated neurodegenerative disorders.Front Pharmacol. 2022 Nov 28;13:1036030. doi: 10.3389/fphar.2022.1036030. eCollection 2022. Front Pharmacol. 2022. PMID: 36518670 Free PMC article.
-
Recent Progress in Regulating the Activity of Enzymes with Photoswitchable Inhibitors.Molecules. 2024 Sep 24;29(19):4523. doi: 10.3390/molecules29194523. Molecules. 2024. PMID: 39407453 Free PMC article. Review.
-
Synthesis, biological evaluation, and computational studies of Tri- and tetracyclic nitrogen-bridgehead compounds as potent dual-acting AChE inhibitors and hH3 receptor antagonists.ACS Chem Neurosci. 2014 Mar 19;5(3):225-42. doi: 10.1021/cn4002126. Epub 2014 Jan 14. ACS Chem Neurosci. 2014. PMID: 24422467 Free PMC article.
-
Recent development of multifunctional agents as potential drug candidates for the treatment of Alzheimer's disease.Curr Med Chem. 2015;22(3):373-404. doi: 10.2174/0929867321666141106122628. Curr Med Chem. 2015. PMID: 25386820 Free PMC article. Review.
References
-
- Ballard C.; Gauthier S.; Corbett A.; Brayne C.; Aarsland D.; Jones E. Alzheimer's disease. Lancet 2011, 377, 1019–1031. - PubMed
-
- Takeda A.; Loveman E.; Clegg A.; Kirby J.; Picot J.; Payne E. C. A systematic review of the clinical effectiveness of donepezil, rivastigmine and galantamine on cognition, quality of life and adverse events in Alzheimer's disease. Int. J. Geriatr. Psychiatry 2006, 21, 17–28. - PubMed
-
- Çokuğraş A. N. Butyrylcholinesterase: Structure and physiological importance. Turk. J. Biochem. 2003, 28, 54–61.
-
- Giacobini E. Cholinergic function and Alzheimer's disease. Int. J. Geriatr. Psychiatry 2003, 18Suppl. 1S1–S5. - PubMed
-
- Giacobini E.Drugs that target cholinesterases. In Cognitive Enhancing Drugs; Buccafusco J. J., Ed.; Birkhäuser: Basel, Boston, Berlin, 2004; pp 11–36.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Miscellaneous

