Disubstituted Bis-THF Moieties as New P2 Ligands in Nonpeptidal HIV-1 Protease Inhibitors
- PMID: 24900331
- PMCID: PMC4018169
- DOI: 10.1021/ml2000356
Disubstituted Bis-THF Moieties as New P2 Ligands in Nonpeptidal HIV-1 Protease Inhibitors
Abstract
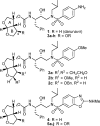
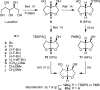
A series of darunavir analogues featuring a substituted bis-THF ring as P2 ligand have been synthesized and evaluated. High affinity protease inhibitors (PIs) with an interesting activity on wild-type HIV and a panel of multi-PI resistant HIV-1 mutants containing clinically observed, primary mutations were identified using a cell-based assay. A number of PIs have been synthesized that show equivalent and greater activity for HIV-1 mutant strains as compared to wild-type HIV-1. The activity on the purified enzyme was confirmed for a selection of analogues.
Keywords: HIV protease; bis-THF bis-diol; darunavir; inhibitors.
Figures
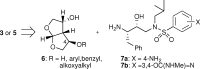
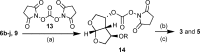
Similar articles
-
Disubstituted Bis-THF Moieties as New P2 Ligands in Nonpeptidal HIV-1 Protease Inhibitors (II).J Med Chem. 2015 May 14;58(9):4029-38. doi: 10.1021/acs.jmedchem.5b00358. Epub 2015 Apr 30. J Med Chem. 2015. PMID: 25897791
-
Novel Protease Inhibitors Containing C-5-Modified bis-Tetrahydrofuranylurethane and Aminobenzothiazole as P2 and P2' Ligands That Exert Potent Antiviral Activity against Highly Multidrug-Resistant HIV-1 with a High Genetic Barrier against the Emergence of Drug Resistance.Antimicrob Agents Chemother. 2019 Jul 25;63(8):e00372-19. doi: 10.1128/AAC.00372-19. Print 2019 Aug. Antimicrob Agents Chemother. 2019. PMID: 31085520 Free PMC article.
-
Design of HIV protease inhibitors targeting protein backbone: an effective strategy for combating drug resistance.Acc Chem Res. 2008 Jan;41(1):78-86. doi: 10.1021/ar7001232. Epub 2007 Aug 28. Acc Chem Res. 2008. PMID: 17722874 Review.
-
Structural Analysis of Potent Hybrid HIV-1 Protease Inhibitors Containing Bis-tetrahydrofuran in a Pseudosymmetric Dipeptide Isostere.J Med Chem. 2020 Aug 13;63(15):8296-8313. doi: 10.1021/acs.jmedchem.0c00529. Epub 2020 Aug 3. J Med Chem. 2020. PMID: 32672965 Free PMC article.
-
Darunavir: a review of its use in the management of HIV infection in adults.Drugs. 2009;69(4):477-503. doi: 10.2165/00003495-200969040-00007. Drugs. 2009. PMID: 19323590 Review.
Cited by
-
Exogenous Nitric Oxide Alleviates the Damage Caused by Tomato Yellow Leaf Curl Virus in Tomato through Regulation of Peptidase Inhibitor Genes.Int J Mol Sci. 2022 Oct 19;23(20):12542. doi: 10.3390/ijms232012542. Int J Mol Sci. 2022. PMID: 36293408 Free PMC article.
-
Recent Progress in the Development of HIV-1 Protease Inhibitors for the Treatment of HIV/AIDS.J Med Chem. 2016 Jun 9;59(11):5172-208. doi: 10.1021/acs.jmedchem.5b01697. Epub 2016 Jan 22. J Med Chem. 2016. PMID: 26799988 Free PMC article. Review.
-
Design, synthesis, biological evaluation and X-ray structural studies of HIV-1 protease inhibitors containing substituted fused-tetrahydropyranyl tetrahydrofuran as P2-ligands.Org Biomol Chem. 2015 Dec 28;13(48):11607-21. doi: 10.1039/c5ob01930c. Epub 2015 Oct 14. Org Biomol Chem. 2015. PMID: 26462551 Free PMC article.
-
Design, Synthesis and X-Ray Structural Studies of Potent HIV-1 Protease Inhibitors Containing C-4 Substituted Tricyclic Hexahydro-Furofuran Derivatives as P2 Ligands.ChemMedChem. 2022 May 4;17(9):e202200058. doi: 10.1002/cmdc.202200058. Epub 2022 Mar 4. ChemMedChem. 2022. PMID: 35170223 Free PMC article.
-
Structure-Based Design of Highly Potent HIV-1 Protease Inhibitors Containing New Tricyclic Ring P2-Ligands: Design, Synthesis, Biological, and X-ray Structural Studies.J Med Chem. 2020 May 14;63(9):4867-4879. doi: 10.1021/acs.jmedchem.0c00202. Epub 2020 Apr 29. J Med Chem. 2020. PMID: 32348139 Free PMC article.
References
-
- Sepkowitz K. A. AIDS—The First 20 Years. N. Engl. J. Med. 2001, 344, 1764–1772. - PubMed
-
- Johnson V. A.; Brun-Vezinet F.; Clotet B.; Gunthard H. F.; Kuritzkes D. R.; Pillay D.; Schapiro J. M.; Richman D. D. Update of the drug resistance mutations in HIV-1: December 2009. Top. HIV Med. 2009, 17, 138–145. - PubMed
-
- Ghosh A. K.; Thompson W. J.; Fitzgerald P. M.; Culberson J. C.; Axel M. G.; McKee S. P.; Huff J. R.; Anderson P. S. Structure-based design of HIV-1 protease inhibitors: Replacement of two amides an a 10π-aromatic system by a fused bis-tetrahydrofuran. J. Med. Chem. 1994, 37, 2506–2508. - PubMed
-
- Surleraux D. L. N. G.; Tahri A.; Verschueren W. G.; Pille G. M. E.; de Kock H. A.; Jonckers T. H. M.; Peeters A.; De Meyer S.; Azijn H.; Pauwels R.; de Bethune M.-P.; King N. M.; Prabu-Jeyabalan M.; Schiffer C. A.; Wigerinck P. B. T. P. Discovery and selection of TMC114, a next generation HIV-1 protease inhibitor. J. Med. Chem. 2005, 48, 1813–1822. - PubMed
LinkOut - more resources
Full Text Sources
Chemical Information
Research Materials
Miscellaneous

