miR-34/449 miRNAs are required for motile ciliogenesis by repressing cp110
- PMID: 24899310
- PMCID: PMC4119886
- DOI: 10.1038/nature13413
miR-34/449 miRNAs are required for motile ciliogenesis by repressing cp110
Abstract
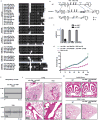
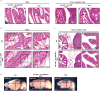
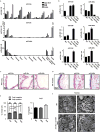
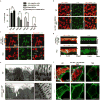
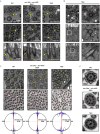
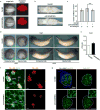
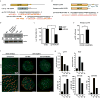
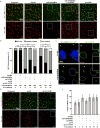
The mir-34/449 family consists of six homologous miRNAs at three genomic loci. Redundancy of miR-34/449 miRNAs and their dominant expression in multiciliated epithelia suggest a functional significance in ciliogenesis. Here we report that mice deficient for all miR-34/449 miRNAs exhibited postnatal mortality, infertility and strong respiratory dysfunction caused by defective mucociliary clearance. In both mouse and Xenopus, miR-34/449-deficient multiciliated cells (MCCs) exhibited a significant decrease in cilia length and number, due to defective basal body maturation and apical docking. The effect of miR-34/449 on ciliogenesis was mediated, at least in part, by post-transcriptional repression of Cp110, a centriolar protein suppressing cilia assembly. Consistent with this, cp110 knockdown in miR-34/449-deficient MCCs restored ciliogenesis by rescuing basal body maturation and docking. Altogether, our findings elucidate conserved cellular and molecular mechanisms through which miR-34/449 regulate motile ciliogenesis.
Figures
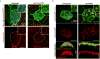

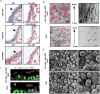

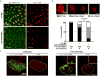
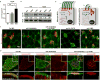
Comment in
-
Cell biology: Short RNAs and shortness of breath.Nature. 2014 Jun 5;510(7503):40-2. doi: 10.1038/510040a. Nature. 2014. PMID: 24899299 Free PMC article.
Similar articles
-
Ciliary transcription factors and miRNAs precisely regulate Cp110 levels required for ciliary adhesions and ciliogenesis.Elife. 2016 Sep 13;5:e17557. doi: 10.7554/eLife.17557. Elife. 2016. PMID: 27623009 Free PMC article.
-
Centrosomal protein CP110 controls maturation of the mother centriole during cilia biogenesis.Development. 2016 May 1;143(9):1491-501. doi: 10.1242/dev.130120. Epub 2016 Mar 10. Development. 2016. PMID: 26965371 Free PMC article.
-
Male infertility-associated Ccdc108 regulates multiciliogenesis via the intraflagellar transport machinery.EMBO Rep. 2022 Apr 5;23(4):e52775. doi: 10.15252/embr.202152775. Epub 2022 Feb 24. EMBO Rep. 2022. PMID: 35201641 Free PMC article.
-
MicroRNAs as key regulators of GTPase-mediated apical actin reorganization in multiciliated epithelia.Small GTPases. 2016 Apr 2;7(2):54-8. doi: 10.1080/21541248.2016.1151099. Epub 2016 May 4. Small GTPases. 2016. PMID: 27144998 Free PMC article. Review.
-
Ciliogenesis and ciliary abnormalities.Med Electron Microsc. 2000;33(3):109-14. doi: 10.1007/s007950000009. Med Electron Microsc. 2000. PMID: 11810467 Review.
Cited by
-
ATP4a is required for development and function of the Xenopus mucociliary epidermis - a potential model to study proton pump inhibitor-associated pneumonia.Dev Biol. 2015 Dec 15;408(2):292-304. doi: 10.1016/j.ydbio.2015.03.013. Epub 2015 Apr 4. Dev Biol. 2015. PMID: 25848696 Free PMC article.
-
LncRNA XIST promotes liver cancer progression by acting as a molecular sponge of miR-200b-3p to regulate ZEB1/2 expression.J Int Med Res. 2021 May;49(5):3000605211016211. doi: 10.1177/03000605211016211. J Int Med Res. 2021. PMID: 34018840 Free PMC article.
-
Motile cilia of the male reproductive system require miR-34/miR-449 for development and function to generate luminal turbulence.Proc Natl Acad Sci U S A. 2019 Feb 26;116(9):3584-3593. doi: 10.1073/pnas.1817018116. Epub 2019 Jan 18. Proc Natl Acad Sci U S A. 2019. PMID: 30659149 Free PMC article.
-
Identification of miR-200c and miR141-Mediated lncRNA-mRNA Crosstalks in Muscle-Invasive Bladder Cancer Subtypes.Front Genet. 2018 Sep 28;9:422. doi: 10.3389/fgene.2018.00422. eCollection 2018. Front Genet. 2018. PMID: 30323832 Free PMC article.
-
Non-oncogenic roles of TAp73: from multiciliogenesis to metabolism.Cell Death Differ. 2018 Jan;25(1):144-153. doi: 10.1038/cdd.2017.178. Epub 2017 Oct 27. Cell Death Differ. 2018. PMID: 29077094 Free PMC article. Review.
References
-
- Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. - PubMed
-
- He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–31. - PubMed
-
- Kim VN. Small RNAs: classification, biogenesis, and function. Mol Cells. 2005;19:1–15. - PubMed
-
- Du T, Zamore PD. microPrimer: the biogenesis and function of microRNA. Development. 2005;132:4645–4652. - PubMed
-
- Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

