Cellular and molecular mechanisms in kidney fibrosis
- PMID: 24892703
- PMCID: PMC4038570
- DOI: 10.1172/JCI72267
Cellular and molecular mechanisms in kidney fibrosis
Abstract
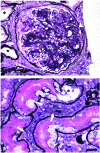
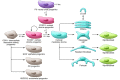
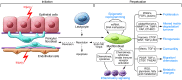
Fibrosis is a characteristic feature of all forms of chronic kidney disease. Deposition of pathological matrix in the interstitial space and within the walls of glomerular capillaries as well as the cellular processes resulting in this deposition are increasingly recognized as important factors amplifying kidney injury and accelerating nephron demise. Recent insights into the cellular and molecular mechanisms of fibrogenesis herald the promise of new therapies to slow kidney disease progression. This review focuses on new findings that enhance understanding of cellular and molecular mechanisms of fibrosis, the characteristics of myofibroblasts, their progenitors, and molecular pathways regulating both fibrogenesis and its resolution.
Figures
Similar articles
-
Origin of myofibroblasts and cellular events triggering fibrosis.Kidney Int. 2015 Feb;87(2):297-307. doi: 10.1038/ki.2014.287. Epub 2014 Aug 27. Kidney Int. 2015. PMID: 25162398 Review.
-
Cellular mechanisms of tissue fibrosis. 3. Novel mechanisms of kidney fibrosis.Am J Physiol Cell Physiol. 2013 Apr 1;304(7):C591-603. doi: 10.1152/ajpcell.00414.2012. Epub 2013 Jan 16. Am J Physiol Cell Physiol. 2013. PMID: 23325411 Free PMC article. Review.
-
[Identification of Biomarkers for Tubular Injury and Interstitial Fibrosis in Chronic Kidney Disease].Yakugaku Zasshi. 2017;137(11):1355-1360. doi: 10.1248/yakushi.17-00150. Yakugaku Zasshi. 2017. PMID: 29093371 Review. Japanese.
-
The role played by perivascular cells in kidney interstitial injury.Clin Nephrol. 2012 May;77(5):400-8. doi: 10.5414/cn107371. Clin Nephrol. 2012. PMID: 22551886 Free PMC article. Review.
-
Decoding myofibroblast origins in human kidney fibrosis.Nature. 2021 Jan;589(7841):281-286. doi: 10.1038/s41586-020-2941-1. Epub 2020 Nov 11. Nature. 2021. PMID: 33176333 Free PMC article.
Cited by
-
Urinary soluble CD90 predicts renal prognosis in patients with diabetic kidney disease.Ann Transl Med. 2021 Feb;9(4):282. doi: 10.21037/atm-20-6528. Ann Transl Med. 2021. PMID: 33708909 Free PMC article.
-
Sonic hedgehog signaling in kidney fibrosis: a master communicator.Sci China Life Sci. 2016 Sep;59(9):920-9. doi: 10.1007/s11427-016-0020-y. Epub 2016 Jun 22. Sci China Life Sci. 2016. PMID: 27333788 Free PMC article. Review.
-
Ion channels as a therapeutic target for renal fibrosis.Front Physiol. 2022 Oct 5;13:1019028. doi: 10.3389/fphys.2022.1019028. eCollection 2022. Front Physiol. 2022. PMID: 36277193 Free PMC article. Review.
-
Beyond EMT: Epithelial STAT3 as a Central Regulator of Fibrogenesis.J Am Soc Nephrol. 2016 Dec;27(12):3502-3504. doi: 10.1681/ASN.2016060603. Epub 2016 Jul 1. J Am Soc Nephrol. 2016. PMID: 27371721 Free PMC article. No abstract available.
-
Yi Qi Qing Re Gao-containing serum inhibits lipopolysaccharide-induced rat mesangial cell proliferation by suppressing the Wnt pathway and TGF-β1 expression.Exp Ther Med. 2016 Apr;11(4):1410-1416. doi: 10.3892/etm.2016.3027. Epub 2016 Jan 28. Exp Ther Med. 2016. PMID: 27073458 Free PMC article.
References
-
- Morel-Maroger Striker L, Killen PD, Chi E, Striker GE. The composition of glomerulosclerosis. I. Studies in focal sclerosis, crescentic glomerulonephritis, and membranoproliferative glomerulonephritis. Lab Invest. 1984;51(2):181–192. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

