Surviving the infarct: A profile of cardiac myosin binding protein-C pathogenicity, diagnostic utility, and proteomics in the ischemic myocardium
- PMID: 24888514
- PMCID: PMC4162529
- DOI: 10.1002/prca.201400011
Surviving the infarct: A profile of cardiac myosin binding protein-C pathogenicity, diagnostic utility, and proteomics in the ischemic myocardium
Abstract
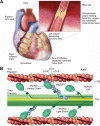
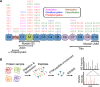
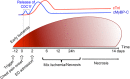
Cardiac myosin binding protein-C (cMyBP-C) is a regulatory protein of the contractile apparatus within the cardiac sarcomere. Ischemic injury to the heart during myocardial infarction (MI) results in the cleavage of cMyBP-C in a phosphorylation-dependent manner and release of an N-terminal fragment (C0C1f) into the circulation. C0C1f has been shown to be pathogenic within cardiac tissue, leading to the development of heart failure. Based on its high levels and early release into the circulation post-MI, C0C1f may serve as a novel biomarker for diagnosing MI more effectively than current clinically used biomarkers. Over time, circulating C0C1f could trigger an autoimmune response leading to myocarditis and progressive cardiac dysfunction. Given the importance of cMyBP-C phosphorylation state in the context of proteolytic cleavage and release into the circulation post-MI, understanding the posttranslational modifications (PTMs) of cMyBP-C would help in further elucidating the role of this protein in health and disease. Accordingly, recent studies have implemented the latest proteomics approaches to define the PTMs of cMyBP-C. The use of such proteomics assays may provide accurate quantitation of the levels of cMyBP-C in the circulation following MI, which could, in turn, demonstrate the efficacy of using plasma cMyBP-C as a cardiac-specific early biomarker of MI. In this review, we define the pathogenic and potential immunogenic effects of C0C1f on cardiac function in the post-MI heart. We also discuss the most advanced proteomics approaches now used to determine cMyBP-C PTMs with the aim of validating C0C1f as an early biomarker of MI.
Keywords: Autoantibodies; Biomarkers; Dilated cardiomyopathy; Myocardial infarction; cMyBP-C.
© The Authors PROTEOMICS - Clinical Applications Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Figures
Similar articles
-
Cardiac myosin binding protein-C is a potential diagnostic biomarker for myocardial infarction.J Mol Cell Cardiol. 2012 Jan;52(1):154-64. doi: 10.1016/j.yjmcc.2011.09.011. Epub 2011 Sep 19. J Mol Cell Cardiol. 2012. PMID: 21971072 Free PMC article.
-
N-terminal fragment of cardiac myosin binding protein-C triggers pro-inflammatory responses in vitro.J Mol Cell Cardiol. 2016 Oct;99:47-56. doi: 10.1016/j.yjmcc.2016.09.003. Epub 2016 Sep 8. J Mol Cell Cardiol. 2016. PMID: 27616755 Free PMC article.
-
Increase in cardiac myosin binding protein-C plasma levels is a sensitive and cardiac-specific biomarker of myocardial infarction.Am J Cardiovasc Dis. 2013 Jun 10;3(2):60-70. Print 2013. Am J Cardiovasc Dis. 2013. PMID: 23785583 Free PMC article.
-
Phosphorylation and function of cardiac myosin binding protein-C in health and disease.J Mol Cell Cardiol. 2010 May;48(5):866-75. doi: 10.1016/j.yjmcc.2009.11.014. Epub 2009 Dec 3. J Mol Cell Cardiol. 2010. PMID: 19962384 Free PMC article. Review.
-
Cardiac myosin binding protein-C as a central target of cardiac sarcomere signaling: a special mini review series.Pflugers Arch. 2014 Feb;466(2):195-200. doi: 10.1007/s00424-013-1396-8. Epub 2013 Nov 7. Pflugers Arch. 2014. PMID: 24196566 Free PMC article. Review.
Cited by
-
The A31P missense mutation in cardiac myosin binding protein C alters protein structure but does not cause haploinsufficiency.Arch Biochem Biophys. 2016 Jul 1;601:133-40. doi: 10.1016/j.abb.2016.01.006. Epub 2016 Jan 9. Arch Biochem Biophys. 2016. PMID: 26777460 Free PMC article.
-
Posttranslational modifications of calcium/calmodulin-dependent protein kinase IIδ and its downstream signaling in human failing hearts.Am J Transl Res. 2017 Aug 15;9(8):3573-3585. eCollection 2017. Am J Transl Res. 2017. PMID: 28861149 Free PMC article.
-
Direct Comparison of Cardiac Myosin-Binding Protein C With Cardiac Troponins for the Early Diagnosis of Acute Myocardial Infarction.Circulation. 2017 Oct 17;136(16):1495-1508. doi: 10.1161/CIRCULATIONAHA.117.028084. Epub 2017 Sep 26. Circulation. 2017. PMID: 28972002 Free PMC article.
-
Sarcomeric protein modification during adrenergic stress enhances cross-bridge kinetics and cardiac output.J Appl Physiol (1985). 2017 Mar 1;122(3):520-530. doi: 10.1152/japplphysiol.00306.2016. Epub 2016 Dec 1. J Appl Physiol (1985). 2017. PMID: 27909224 Free PMC article.
-
Amino terminus of cardiac myosin binding protein-C regulates cardiac contractility.J Mol Cell Cardiol. 2021 Jul;156:33-44. doi: 10.1016/j.yjmcc.2021.03.009. Epub 2021 Mar 26. J Mol Cell Cardiol. 2021. PMID: 33781820 Free PMC article.
References
-
- Ahmad M. Biomarkers in acute myocardial infarction. J. Clin. Exp. 2012;3:222.
-
- Reichlin T, Hochholzer W, Bassetti S, Steuer S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N. Engl. J. Med. 2009;361:858–867. - PubMed
-
- Thygesen K, Alpert JS, Jaffe AS, Simoons ML, et al. Third universal definition of myocardial infarction. J. Am. Coll. Cardiol. 2012;60:1581–1598. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

