Synergistic white matter protection with acute-on-chronic endotoxin and subsequent asphyxia in preterm fetal sheep
- PMID: 24886663
- PMCID: PMC4039331
- DOI: 10.1186/1742-2094-11-89
Synergistic white matter protection with acute-on-chronic endotoxin and subsequent asphyxia in preterm fetal sheep
Abstract
Background: Perinatal asphyxia and exposure to intrauterine infection are associated with impaired neurodevelopment in preterm infants. Acute exposure to non-injurious infection and/or inflammation can either protect or sensitize the brain to subsequent hypoxia-ischemia. However, the effects of subacute infection and/or inflammation are unclear. In this study we tested the hypothesis that acute-on-chronic exposure to lipopolysaccharide (LPS) would exacerbate white matter injury after subsequent asphyxia in preterm fetal sheep.
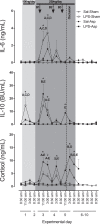
Methods: Fetal sheep at 0.7 gestational age received a continuous LPS infusion at 100 ng/kg for 24 hours, then 250 ng/kg/24 hours for 96 hours, plus 1 μg boluses of LPS at 48, 72, and 96 hours or the same volume of saline. Four hours after the last bolus, complete umbilical cord occlusion or sham occlusion was induced for 15 minutes. Sheep were sacrificed 10 days after the start of infusions.
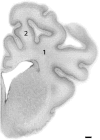
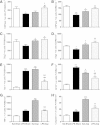
Results: LPS exposure was associated with induction of microglia and astrocytes and loss of total and immature and mature oligodendrocytes (n = 9) compared to sham controls (n = 9). Umbilical cord occlusion with saline infusions was associated with induction of microglia, astrogliosis, and loss of immature and mature oligodendrocytes (n = 9). LPS exposure before asphyxia (n = 8) was associated with significantly reduced microglial activation and astrogliosis and improved numbers of immature and mature oligodendrocytes compared to either LPS exposure or asphyxia alone.
Conclusions: Contrary to our initial hypothesis, the combination of acute-on-chronic LPS with subsequent asphyxia reduced neuroinflammation and white matter injury compared with either intervention alone.
Figures


Similar articles
-
Acute on chronic exposure to endotoxin in preterm fetal sheep.Am J Physiol Regul Integr Comp Physiol. 2013 Feb;304(3):R189-97. doi: 10.1152/ajpregu.00388.2012. Epub 2012 Dec 12. Am J Physiol Regul Integr Comp Physiol. 2013. PMID: 23235324
-
Acute on chronic exposure to endotoxin is associated with enhanced chemoreflex responses in preterm fetal sheep.Am J Physiol Regul Integr Comp Physiol. 2013 May 15;304(10):R799-803. doi: 10.1152/ajpregu.00005.2013. Epub 2013 Mar 13. Am J Physiol Regul Integr Comp Physiol. 2013. PMID: 23485869
-
Protective effects of delayed intraventricular TLR7 agonist administration on cerebral white and gray matter following asphyxia in the preterm fetal sheep.Sci Rep. 2019 Jul 2;9(1):9562. doi: 10.1038/s41598-019-45872-y. Sci Rep. 2019. PMID: 31267031 Free PMC article.
-
Models of white matter injury: comparison of infectious, hypoxic-ischemic, and excitotoxic insults.Ment Retard Dev Disabil Res Rev. 2002;8(1):30-8. doi: 10.1002/mrdd.10007. Ment Retard Dev Disabil Res Rev. 2002. PMID: 11921384 Review.
-
Therapeutic Strategies for Leukodystrophic Disorders Resulting from Perinatal Asphyxia: Focus on Myelinating Oligodendrocytes.Mol Neurobiol. 2018 May;55(5):4388-4402. doi: 10.1007/s12035-017-0647-7. Epub 2017 Jun 28. Mol Neurobiol. 2018. PMID: 28660484 Free PMC article. Review.
Cited by
-
Instrumentation of Near-term Fetal Sheep for Multivariate Chronic Non-anesthetized Recordings.J Vis Exp. 2015 Oct 25;(105):e52581. doi: 10.3791/52581. J Vis Exp. 2015. PMID: 26555084 Free PMC article.
-
Amniotic LPS-Induced Apoptosis in the Fetal Brain Is Suppressed by Vaginal LPS Preconditioning but Is Promoted by Continuous Ischemic Reperfusion.Int J Mol Sci. 2022 Feb 4;23(3):1787. doi: 10.3390/ijms23031787. Int J Mol Sci. 2022. PMID: 35163709 Free PMC article.
-
A Systematic Review of Magnesium Sulfate for Perinatal Neuroprotection: What Have We Learnt From the Past Decade?Front Neurol. 2020 May 27;11:449. doi: 10.3389/fneur.2020.00449. eCollection 2020. Front Neurol. 2020. PMID: 32536903 Free PMC article.
-
Magnetic Resonance Imaging Correlates of White Matter Gliosis and Injury in Preterm Fetal Sheep Exposed to Progressive Systemic Inflammation.Int J Mol Sci. 2020 Nov 24;21(23):8891. doi: 10.3390/ijms21238891. Int J Mol Sci. 2020. PMID: 33255257 Free PMC article.
-
Preclinical Models of Encephalopathy of Prematurity.Dev Neurosci. 2015;37(4-5):277-88. doi: 10.1159/000371721. Epub 2015 Feb 18. Dev Neurosci. 2015. PMID: 25722056 Free PMC article. Review.
References
-
- Behrman RE, Butler AS, editor. Committee on Understanding Premature Birth and Assuring Healthy Outcomes. Preterm Birth: Causes, Consequences, and Prevention. Institute of Medicine, The National Academies Press: Washington, D.C; 2007. http://books.nap.edu//catalog/11622.html#toc. - PubMed
-
- Buser JR, Maire J, Riddle A, Gong X, Nguyen T, Nelson K, Luo NL, Ren J, Struve J, Sherman LS, Miller SP, Chau V, Hendson G, Ballabh P, Grafe MR, Back SA. Arrested preoligodendrocyte maturation contributes to myelination failure in premature infants. Ann Neurol. 2012;71:93–109. doi: 10.1002/ana.22627. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

