Clinical experience with ipilimumab 3 mg/kg: real-world efficacy and safety data from an expanded access programme cohort
- PMID: 24885479
- PMCID: PMC4030525
- DOI: 10.1186/1479-5876-12-116
Clinical experience with ipilimumab 3 mg/kg: real-world efficacy and safety data from an expanded access programme cohort
Abstract
Background: Ipilimumab improves survival in patients with advanced melanoma. The activity and safety of ipilimumab outside of a clinical trial was assessed in an expanded access programme (EAP).
Methods: Ipilimumab was available upon physician request for patients aged 16 or over with pretreated stage III (unresectable)/IV melanoma, for whom no other therapeutic option was available. Patients received ipilimumab 3 mg/kg every 3 weeks for four doses. Patients with stable disease or an objective response to ipilimumab were eligible for retreatment upon disease progression. Tumour assessments were conducted at baseline and week 12. Patients were monitored for adverse events (AEs) within 3 to 4 days of each scheduled visit.
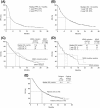
Results: Of 855 patients participating in the EAP in Italy, 833 were evaluable for response. Of these, 13% had an objective immune response, and the immune-related disease control rate was 34%. Median progression-free survival and overall survival were 3.7 and 7.2 months, respectively. Efficacy was independent of BRAF and NRAS mutational status. Overall, 33% of patients reported an immune-related AE (irAE). The frequency of irAEs was not associated with response to ipilimumab.
Conclusions: Outside of a clinical trial setting, ipilimumab is a feasible treatment option in patients with pretreated metastatic melanoma, regardless of BRAF and NRAS mutational status. Data from this large cohort of patients support clinical trial evidence that ipilimumab can induce durable disease control and long-term survival in patients who have failed to respond to prior treatment.
Figures
Similar articles
-
Efficacy and safety of ipilimumab 3mg/kg in patients with pretreated, metastatic, mucosal melanoma.Eur J Cancer. 2014 Jan;50(1):121-7. doi: 10.1016/j.ejca.2013.09.007. Epub 2013 Oct 4. Eur J Cancer. 2014. PMID: 24100024
-
Efficacy and safety of ipilimumab in elderly patients with pretreated advanced melanoma treated at Italian centres through the expanded access programme.J Exp Clin Cancer Res. 2014 Apr 4;33(1):30. doi: 10.1186/1756-9966-33-30. J Exp Clin Cancer Res. 2014. PMID: 24708900 Free PMC article. Clinical Trial.
-
Clinical experience with ipilimumab 10 mg/kg in patients with melanoma treated at Italian centres as part of a European expanded access programme.J Exp Clin Cancer Res. 2013 Oct 25;32(1):82. doi: 10.1186/1756-9966-32-82. J Exp Clin Cancer Res. 2013. PMID: 24423086 Free PMC article.
-
Anti-CTLA4 monoclonal antibody Ipilimumab in the treatment of metastatic melanoma: recent findings.Recent Pat Anticancer Drug Discov. 2008 Jun;3(2):105-13. doi: 10.2174/157489208784638767. Recent Pat Anticancer Drug Discov. 2008. PMID: 18537753 Review.
-
Concurrent radiotherapy and ipilimumab immunotherapy for patients with melanoma.Cancer Immunol Res. 2013 Aug;1(2):92-8. doi: 10.1158/2326-6066.CIR-13-0082. Epub 2013 Jul 25. Cancer Immunol Res. 2013. PMID: 24777500 Free PMC article. Review.
Cited by
-
The efficacy and safety of nivolumab in the treatment of advanced melanoma: a meta-analysis of clinical trials.Onco Targets Ther. 2016 Mar 16;9:1571-8. doi: 10.2147/OTT.S96762. eCollection 2016. Onco Targets Ther. 2016. PMID: 27051297 Free PMC article.
-
Pre-treatment comorbidities, C-reactive protein and eosinophil count, and immune-related adverse events as predictors of survival with checkpoint inhibition for multiple tumour entities.Cancer Med. 2023 Jun;12(11):12253-12262. doi: 10.1002/cam4.5919. Epub 2023 Apr 21. Cancer Med. 2023. PMID: 37084178 Free PMC article.
-
Association of CTLA-4 Gene Variants with Response to Therapy and Long-term Survival in Metastatic Melanoma Patients Treated with Ipilimumab: An Italian Melanoma Intergroup Study.Front Immunol. 2017 Apr 12;8:386. doi: 10.3389/fimmu.2017.00386. eCollection 2017. Front Immunol. 2017. PMID: 28446908 Free PMC article.
-
Immune-related adverse events: promising predictors for efficacy of immune checkpoint inhibitors.Cancer Immunol Immunother. 2021 Sep;70(9):2559-2576. doi: 10.1007/s00262-020-02803-5. Epub 2021 Feb 12. Cancer Immunol Immunother. 2021. PMID: 33576872 Free PMC article. Review.
-
Checkpoint inhibitor immunotherapy toxicity and overall survival among older adults with advanced cancer.J Geriatr Oncol. 2021 Jun;12(5):813-819. doi: 10.1016/j.jgo.2021.02.002. Epub 2021 Feb 21. J Geriatr Oncol. 2021. PMID: 33627226 Free PMC article.
References
-
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. - DOI - PMC - PubMed
-
- Robert C, Thomas L, Bondarenko I, O'Day S, JW MD, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, Davidson N, Richards J, Maio M, Hauschild A, Miller WH Jr, Gascon P, Lotem M, Harmankaya K, Ibrahim R, Francis S, Chen TT, Humphrey R, Hoos A, Wolchok JD. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. - DOI - PubMed
-
- Lebbé C, Weber JS, Maio M, Neyns B, Harmankaya K, Hamid O, O'Day S, Chin KM, Opatt McDowell D, Cykowski L, McHenry B, Wolchok JD. Long-term survival in patients with metastatic melanoma who received ipilimumab in four phase II trials [abstract] J Clin Oncol. 2013;31(suppl):9053.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

