Ablation of MMP9 gene ameliorates paracellular permeability and fibrinogen-amyloid beta complex formation during hyperhomocysteinemia
- PMID: 24865997
- PMCID: PMC4158659
- DOI: 10.1038/jcbfm.2014.102
Ablation of MMP9 gene ameliorates paracellular permeability and fibrinogen-amyloid beta complex formation during hyperhomocysteinemia
Abstract
Increased blood level of homocysteine (Hcy), called hyperhomocysteinemia (HHcy) accompanies many cognitive disorders including Alzheimer's disease. We hypothesized that HHcy-enhanced cerebrovascular permeability occurs via activation of matrix metalloproteinase-9 (MMP9) and leads to an increased formation of fibrinogen-β-amyloid (Fg-Aβ) complex. Cerebrovascular permeability changes were assessed in C57BL/6J (wild type, WT), cystathionine-β-synthase heterozygote (Cbs+/-, a genetic model of HHcy), MMP9 gene knockout (Mmp9-/-), and Cbs and Mmp9 double knockout (Cbs+/-/Mmp9-/-) mice using a dual-tracer probing method. Expression of vascular endothelial cadherin (VE-cadherin) and Fg-Aβ complex formation was assessed in mouse brain cryosections by immunohistochemistry. Short-term memory of mice was assessed with a novel object recognition test. The cerebrovascular permeability in Cbs+/- mice was increased via mainly the paracellular transport pathway. VE-cadherin expression was the lowest and Fg-Aβ complex formation was the highest along with the diminished short-term memory in Cbs+/- mice. These effects of HHcy were ameliorated in Cbs+/-/Mmp9-/- mice. Thus, HHcy causes activation of MMP9 increasing cerebrovascular permeability by downregulation of VE-cadherin resulting in an enhanced formation of Fg-Aβ complex that can be associated with loss of memory. These data may lead to the identification of new targets for therapeutic intervention that can modulate HHcy-induced cerebrovascular permeability and resultant pathologies.
Figures
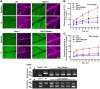

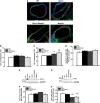


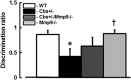
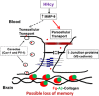
Similar articles
-
Hydrogen Sulfide Ameliorates Homocysteine-Induced Alzheimer's Disease-Like Pathology, Blood-Brain Barrier Disruption, and Synaptic Disorder.Mol Neurobiol. 2016 May;53(4):2451-2467. doi: 10.1007/s12035-015-9212-4. Epub 2015 May 28. Mol Neurobiol. 2016. PMID: 26019015 Free PMC article.
-
Cerebrovascular disorders caused by hyperfibrinogenaemia.J Physiol. 2016 Oct 15;594(20):5941-5957. doi: 10.1113/JP272558. Epub 2016 Jun 16. J Physiol. 2016. PMID: 27121987 Free PMC article.
-
Hydrogen sulfide ameliorates hyperhomocysteinemia-associated chronic renal failure.Am J Physiol Renal Physiol. 2009 Aug;297(2):F410-9. doi: 10.1152/ajprenal.00145.2009. Epub 2009 May 27. Am J Physiol Renal Physiol. 2009. PMID: 19474193 Free PMC article.
-
The pathophysiological hypothesis of homocysteine thiolactone-mediated vascular disease.J Physiol Pharmacol. 2008 Dec;59 Suppl 9:155-67. J Physiol Pharmacol. 2008. PMID: 19261978 Review.
-
Mechanisms of cardiovascular remodeling in hyperhomocysteinemia.Antioxid Redox Signal. 2011 Oct 1;15(7):1927-43. doi: 10.1089/ars.2010.3721. Epub 2011 Apr 21. Antioxid Redox Signal. 2011. PMID: 21126196 Free PMC article. Review.
Cited by
-
Sex-specific implications of inflammation in covert cerebral small vessel disease.BMC Neurol. 2024 Jun 27;24(1):220. doi: 10.1186/s12883-024-03730-z. BMC Neurol. 2024. PMID: 38937678 Free PMC article.
-
Hyperhomocysteinemia exacerbates Alzheimer's disease pathology by way of the β-amyloid fibrinogen interaction.J Thromb Haemost. 2016 Jul;14(7):1442-52. doi: 10.1111/jth.13340. Epub 2016 Jun 13. J Thromb Haemost. 2016. PMID: 27090576 Free PMC article.
-
The blood-brain barrier in Alzheimer's disease.Neurobiol Dis. 2017 Nov;107:41-56. doi: 10.1016/j.nbd.2016.07.007. Epub 2016 Jul 15. Neurobiol Dis. 2017. PMID: 27425887 Free PMC article. Review.
-
Hyperfibrinogenemia-mediated astrocyte activation.Brain Res. 2018 Nov 15;1699:158-165. doi: 10.1016/j.brainres.2018.08.023. Epub 2018 Aug 25. Brain Res. 2018. PMID: 30153459 Free PMC article.
-
Vascular and non-vascular contributors to memory reduction during traumatic brain injury.Eur J Neurosci. 2019 Sep;50(5):2860-2876. doi: 10.1111/ejn.14390. Epub 2019 Mar 12. Eur J Neurosci. 2019. PMID: 30793398 Free PMC article. Review.
References
-
- Tyagi S. Homocyst(e)ine and heart disease: pathophysiology of extracellular matrix. Clin Exp Hypertens. 1999;21:181–198. - PubMed
-
- Woo KS, Chook P, Lolin YI, Cheung ASP, Chan LT, Sun YY, et al. Hyperhomocyst(e)inemia is a risk factor for arterial endothelial dysfunction in humans. Circulation. 1997;96:2542–2544. - PubMed
-
- Lawrence de Koning AB, Werstuck GH, Zhou J, Austin RC. Hyperhomocysteinemia and its role in the development of atherosclerosis. Clin Biochem. 2003;36:431–441. - PubMed
-
- van Beynum IM, Smeitink JAM, den Heijer M, te Poele Pothoff MTWB, Blom HJ. Hyperhomocysteinemia: a risk factor for ischemic stroke in children. Circulation. 1999;99:2070–2072. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

