Directed differentiation of human induced pluripotent stem cells toward bone and cartilage: in vitro versus in vivo assays
- PMID: 24855277
- PMCID: PMC4073820
- DOI: 10.5966/sctm.2013-0154
Directed differentiation of human induced pluripotent stem cells toward bone and cartilage: in vitro versus in vivo assays
Abstract
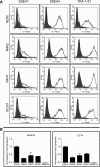
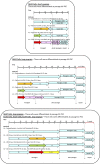
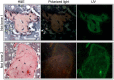
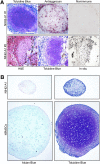
The ability to differentiate induced pluripotent stem cells (iPSCs) into committed skeletal progenitors could allow for an unlimited autologous supply of such cells for therapeutic uses; therefore, we attempted to create novel bone-forming cells from human iPSCs using lines from two distinct tissue sources and methods of differentiation that we previously devised for osteogenic differentiation of human embryonic stem cells, and as suggested by other publications. The resulting cells were assayed using in vitro methods, and the results were compared with those obtained from in vivo transplantation assays. Our results show that true bone was formed in vivo by derivatives of several iPSC lines, but that the successful cell lines and differentiation methodologies were not predicted by the results of the in vitro assays. In addition, bone was formed equally well from iPSCs originating from skin or bone marrow stromal cells (also known as bone marrow-derived mesenchymal stem cells), suggesting that the iPSCs did not retain a "memory" of their previous life. Furthermore, one of the iPSC-derived cell lines formed verifiable cartilage in vivo, which likewise was not predicted by in vitro assays.
Keywords: Bone; Chondrogenesis; Induced pluripotent stem cells; Osteoblast; Transplantation.
©AlphaMed Press.
Figures


Similar articles
-
Footprint-free human induced pluripotent stem cells from articular cartilage with redifferentiation capacity: a first step toward a clinical-grade cell source.Stem Cells Transl Med. 2014 Apr;3(4):433-47. doi: 10.5966/sctm.2013-0138. Epub 2014 Mar 6. Stem Cells Transl Med. 2014. PMID: 24604283 Free PMC article.
-
Conditioned Medium Enhances Osteogenic Differentiation of Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells.Tissue Eng Regen Med. 2019 Jan 29;16(2):141-150. doi: 10.1007/s13770-018-0173-3. eCollection 2019 Apr. Tissue Eng Regen Med. 2019. PMID: 30989041 Free PMC article.
-
Cartilage from human-induced pluripotent stem cells: comparison with neo-cartilage from chondrocytes and bone marrow mesenchymal stromal cells.Cell Tissue Res. 2021 Nov;386(2):309-320. doi: 10.1007/s00441-021-03498-5. Epub 2021 Jul 9. Cell Tissue Res. 2021. PMID: 34241697 Free PMC article.
-
Small Molecule Regulation of Stem Cells that Generate Bone, Chondrocyte, and Cardiac Cells.Curr Top Med Chem. 2020;20(26):2344-2361. doi: 10.2174/1568026620666200820143912. Curr Top Med Chem. 2020. PMID: 32819246 Review.
-
iPSCs chondrogenic differentiation for personalized regenerative medicine: a literature review.Stem Cell Res Ther. 2024 Jun 26;15(1):185. doi: 10.1186/s13287-024-03794-1. Stem Cell Res Ther. 2024. PMID: 38926793 Free PMC article. Review.
Cited by
-
Pluripotent stem cells as a source of osteoblasts for bone tissue regeneration.Biomaterials. 2019 Mar;196:31-45. doi: 10.1016/j.biomaterials.2018.02.009. Epub 2018 Feb 5. Biomaterials. 2019. PMID: 29456164 Free PMC article.
-
Current Therapeutic Strategies for Stem Cell-Based Cartilage Regeneration.Stem Cells Int. 2018 Mar 25;2018:8490489. doi: 10.1155/2018/8490489. eCollection 2018. Stem Cells Int. 2018. PMID: 29765426 Free PMC article. Review.
-
Combinatorial cassettes to systematically evaluate tissue-engineered constructs in recipient mice.Biomaterials. 2018 Dec;186:31-43. doi: 10.1016/j.biomaterials.2018.09.035. Epub 2018 Sep 24. Biomaterials. 2018. PMID: 30278344 Free PMC article.
-
Pathways to understanding the genomic aetiology of osteoarthritis.Hum Mol Genet. 2017 Oct 1;26(R2):R193-R201. doi: 10.1093/hmg/ddx302. Hum Mol Genet. 2017. PMID: 28977450 Free PMC article. Review.
-
Improved approach for chondrogenic differentiation of human induced pluripotent stem cells.Stem Cell Rev Rep. 2015 Apr;11(2):242-53. doi: 10.1007/s12015-014-9581-5. Stem Cell Rev Rep. 2015. PMID: 25578634 Free PMC article.
References
-
- Buttery LD, Bourne S, Xynos JD, et al. Differentiation of osteoblasts and in vitro bone formation from murine embryonic stem cells. Tissue Eng. 2001;7:89–99. - PubMed
-
- Ahn SE, Kim S, Park KH, et al. Primary bone-derived cells induce osteogenic differentiation without exogenous factors in human embryonic stem cells. Biochem Biophys Res Commun. 2006;340:403–408. - PubMed
-
- Kärner E, Unger C, Sloan AJ, et al. Bone matrix formation in osteogenic cultures derived from human embryonic stem cells in vitro. Stem Cells Dev. 2007;16:39–52. - PubMed
-
- Toh WS, Yang Z, Liu H, et al. Effects of culture conditions and bone morphogenetic protein 2 on extent of chondrogenesis from human embryonic stem cells. Stem Cells. 2007;25:950–960. - PubMed
-
- Kim S, Kim SS, Lee SH, et al. In vivo bone formation from human embryonic stem cell-derived osteogenic cells in poly(d,l-lactic-co-glycolic acid)/hydroxyapatite composite scaffolds. Biomaterials. 2008;29:1043–1053. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

