S100/Calgranulin-mediated inflammation accelerates left ventricular hypertrophy and aortic valve sclerosis in chronic kidney disease in a receptor for advanced glycation end products-dependent manner
- PMID: 24855059
- PMCID: PMC4108264
- DOI: 10.1161/ATVBAHA.114.303508
S100/Calgranulin-mediated inflammation accelerates left ventricular hypertrophy and aortic valve sclerosis in chronic kidney disease in a receptor for advanced glycation end products-dependent manner
Abstract
Objective: S100A12 and fibroblast growth factor 23 are biomarkers of cardiovascular morbidity and mortality in patients with chronic kidney disease (CKD). We tested the hypothesis that human S100/calgranulin would accelerate cardiovascular disease in mice subjected to CKD.
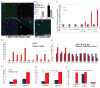
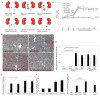
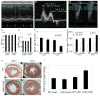
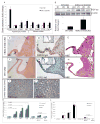
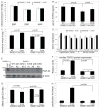
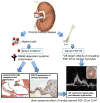
Approach and results: A bacterial artificial chromosome of the human S100/calgranulin gene cluster containing the genes and regulatory elements for S100A8, S100A9, and S100A12 was expressed in C57BL/6J mouse (hBAC-S100) to generate a novel humanized mouse model. CKD was induced by ureteral ligation, and hBAC-S100 mice and wild-type mice were studied after 10 weeks of chronic uremia. hBAC-S100 mice with CKD showed increased fibroblast growth factor 23 in the hearts, left ventricular hypertrophy, diastolic dysfunction, focal cartilaginous metaplasia, and calcification of the mitral and aortic valve annulus together with aortic valve sclerosis. This phenotype was not observed in wild-type mice with CKD or in hBAC-S100 mice lacking the receptor for advanced glycation end products with CKD, suggesting that the inflammatory milieu mediated by S100/receptor for advanced glycation end products promotes pathological cardiac hypertrophy in CKD. In vitro, inflammatory stimuli including interleukin-6, tumor necrosis factor-α, lipopolysaccarides, or serum from hBAC-S100 mice upregulated fibroblast growth factor 23 mRNA and protein in primary murine neonatal and adult cardiac fibroblasts.
Conclusions: Myeloid-derived human S100/calgranulin is associated with the development of cardiac hypertrophy and ectopic cardiac calcification in a receptor for advanced glycation end products-dependent manner in a mouse model of CKD. We speculate that fibroblast growth factor 23 produced by cardiac fibroblasts in response to cytokines may act in a paracrine manner to accelerate left ventricular hypertrophy and diastolic dysfunction in hBAC-S100 mice with CKD.
Keywords: S100 proteins; glycosylation end products, advanced; hypertrophy, left ventricular; renal insufficiency, chronic.
© 2014 American Heart Association, Inc.
Conflict of interest statement
Figures

Similar articles
-
Chronic sustained inflammation links to left ventricular hypertrophy and aortic valve sclerosis: a new link between S100/RAGE and FGF23.Inflamm Cell Signal. 2014;1(5):e279. doi: 10.14800/ics.279. Inflamm Cell Signal. 2014. PMID: 26082935 Free PMC article.
-
IL-22 is induced by S100/calgranulin and impairs cholesterol efflux in macrophages by downregulating ABCG1.J Lipid Res. 2014 Mar;55(3):443-54. doi: 10.1194/jlr.M044305. Epub 2013 Dec 23. J Lipid Res. 2014. PMID: 24367046 Free PMC article.
-
S100A12 and the S100/Calgranulins: Emerging Biomarkers for Atherosclerosis and Possibly Therapeutic Targets.Arterioscler Thromb Vasc Biol. 2015 Dec;35(12):2496-507. doi: 10.1161/ATVBAHA.115.302072. Epub 2015 Oct 29. Arterioscler Thromb Vasc Biol. 2015. PMID: 26515415 Free PMC article. Review.
-
S100a8/9 (S100 Calcium Binding Protein a8/9) Promotes Cardiac Hypertrophy Via Upregulation of FGF23 (Fibroblast Growth Factor 23) in Mice.J Am Heart Assoc. 2024 May 21;13(10):e028006. doi: 10.1161/JAHA.122.028006. Epub 2024 May 10. J Am Heart Assoc. 2024. PMID: 38726894 Free PMC article.
-
Phagocyte-specific S100 proteins: a novel group of proinflammatory molecules.Trends Immunol. 2003 Apr;24(4):155-8. doi: 10.1016/s1471-4906(03)00062-0. Trends Immunol. 2003. PMID: 12697438 Review.
Cited by
-
Cardiac actions of fibroblast growth factor 23.Bone. 2017 Jul;100:69-79. doi: 10.1016/j.bone.2016.10.001. Epub 2016 Oct 7. Bone. 2017. PMID: 27725315 Free PMC article. Review.
-
Local and systemic RAGE axis changes in pulmonary hypertension: CTEPH and iPAH.PLoS One. 2014 Sep 4;9(9):e106440. doi: 10.1371/journal.pone.0106440. eCollection 2014. PLoS One. 2014. PMID: 25188497 Free PMC article.
-
sRAGE attenuates angiotensin II-induced cardiomyocyte hypertrophy by inhibiting RAGE-NFκB-NLRP3 activation.Inflamm Res. 2018 Aug;67(8):691-701. doi: 10.1007/s00011-018-1160-9. Epub 2018 May 23. Inflamm Res. 2018. PMID: 29796842
-
Deregulated Renal Calcium and Phosphate Transport during Experimental Kidney Failure.PLoS One. 2015 Nov 13;10(11):e0142510. doi: 10.1371/journal.pone.0142510. eCollection 2015. PLoS One. 2015. PMID: 26566277 Free PMC article.
-
S100A8/A9 and sRAGE kinetic after polytrauma; an explorative observational study.Scand J Trauma Resusc Emerg Med. 2017 Nov 25;25(1):114. doi: 10.1186/s13049-017-0455-0. Scand J Trauma Resusc Emerg Med. 2017. PMID: 29178941 Free PMC article.
References
-
- Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. The New England Journal of Medicine. 2004;351:1296–1305. - PubMed
-
- Mori Y, Kosaki A, Kishimoto N, Kimura T, Iida K, Fukui M, Nakajima F, Nagahara M, Urakami M, Iwasaka T, Matsubara H. Increased plasma s100a12 (en-rage) levels in hemodialysis patients with atherosclerosis. Am J Nephrol. 2009;29:18–24. - PubMed
-
- Kim JK, Park S, Lee MJ, Song YR, Han SH, Kim SG, Kang SW, Choi KH, Kim HJ, Yoo TH. Plasma levels of soluble receptor for advanced glycation end products (srage) and proinflammatory ligand for rage (en-rage) are associated with carotid atherosclerosis in patients with peritoneal dialysis. Atherosclerosis. 2012;220:208–214. - PubMed
-
- Nakashima A, Carrero JJ, Qureshi AR, Miyamoto T, Anderstam B, Barany P, Heimburger O, Stenvinkel P, Lindholm B. Effect of circulating soluble receptor for advanced glycation end products (srage) and the proinflammatory rage ligand (en-rage, s100a12) on mortality in hemodialysis patients. Clin J Am Soc Nephrol. 2010;12:2213–2219. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

