G-protein-coupled receptors, Hedgehog signaling and primary cilia
- PMID: 24845016
- PMCID: PMC4130902
- DOI: 10.1016/j.semcdb.2014.05.002
G-protein-coupled receptors, Hedgehog signaling and primary cilia
Abstract
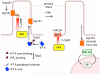
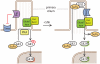
The Hedgehog (Hh) pathway has become an important model to study the cell biology of primary cilia, and reciprocally, the study of ciliary processes provides an opportunity to solve longstanding mysteries in the mechanism of vertebrate Hh signal transduction. The cilium is emerging as an unique compartment for G-protein-coupled receptor (GPCR) signaling in many systems. Two members of the GPCR family, Smoothened and Gpr161, play important roles in the Hh pathway. We review the current understanding of how these proteins may function to regulate Hh signaling and also highlight some of the critical unanswered questions being tackled by the field. Uncovering GPCR-regulated mechanisms important in Hh signaling may provide therapeutic strategies against the Hh pathway that plays important roles in development, regeneration and cancer.
Keywords: G-protein coupled receptors; Hedgehog; Primary cilia; Protein Kinase A.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
The primary cilium as a Hedgehog signal transduction machine.Methods Cell Biol. 2009;94:199-222. doi: 10.1016/S0091-679X(08)94010-3. Epub 2009 Dec 23. Methods Cell Biol. 2009. PMID: 20362092 Free PMC article.
-
The intrahepatic signalling niche of hedgehog is defined by primary cilia positive cells during chronic liver injury.J Hepatol. 2014 Jan;60(1):143-51. doi: 10.1016/j.jhep.2013.08.012. Epub 2013 Aug 23. J Hepatol. 2014. PMID: 23978713
-
G-protein-coupled receptors and localized signaling in the primary cilium during ventral neural tube patterning.Birth Defects Res A Clin Mol Teratol. 2015 Jan;103(1):12-9. doi: 10.1002/bdra.23267. Epub 2014 Jun 11. Birth Defects Res A Clin Mol Teratol. 2015. PMID: 24917297
-
[Progress on Hedgehog signaling transduction].Sheng Li Xue Bao. 2014 Aug 25;66(4):415-22. Sheng Li Xue Bao. 2014. PMID: 25131782 Review. Chinese.
-
Signaling in the primary cilium through the lens of the Hedgehog pathway.Wiley Interdiscip Rev Dev Biol. 2020 Nov;9(6):e377. doi: 10.1002/wdev.377. Epub 2020 Feb 21. Wiley Interdiscip Rev Dev Biol. 2020. PMID: 32084300 Free PMC article. Review.
Cited by
-
Paralog-specific TTC30 regulation of Sonic hedgehog signaling.Front Mol Biosci. 2023 Nov 23;10:1268722. doi: 10.3389/fmolb.2023.1268722. eCollection 2023. Front Mol Biosci. 2023. PMID: 38074101 Free PMC article.
-
Formation of the B9-domain protein complex MKS1-B9D2-B9D1 is essential as a diffusion barrier for ciliary membrane proteins.Mol Biol Cell. 2020 Sep 15;31(20):2259-2268. doi: 10.1091/mbc.E20-03-0208. Epub 2020 Jul 29. Mol Biol Cell. 2020. PMID: 32726168 Free PMC article.
-
Regulation of the oncoprotein Smoothened by small molecules.Nat Chem Biol. 2015 Apr;11(4):246-55. doi: 10.1038/nchembio.1776. Nat Chem Biol. 2015. PMID: 25785427 Review.
-
Methods for Studying Ciliary Import Mechanisms.Methods Mol Biol. 2016;1454:1-14. doi: 10.1007/978-1-4939-3789-9_1. Methods Mol Biol. 2016. PMID: 27514912 Free PMC article.
-
Regulation of Sufu activity by p66β and Mycbp provides new insight into vertebrate Hedgehog signaling.Genes Dev. 2014 Nov 15;28(22):2547-63. doi: 10.1101/gad.249425.114. Genes Dev. 2014. PMID: 25403183 Free PMC article.
References
-
- Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–87. - PubMed
-
- Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–376. - PubMed
-
- Hammerschmidt M, Bitgood MJ, McMahon AP. Protein kinase A is a common negative regulator of Hedgehog signaling in the vertebrate embryo. Genes Dev. 1996;10:647–658. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

