Removing T-cell epitopes with computational protein design
- PMID: 24843166
- PMCID: PMC4060723
- DOI: 10.1073/pnas.1321126111
Removing T-cell epitopes with computational protein design
Abstract
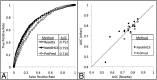
Immune responses can make protein therapeutics ineffective or even dangerous. We describe a general computational protein design method for reducing immunogenicity by eliminating known and predicted T-cell epitopes and maximizing the content of human peptide sequences without disrupting protein structure and function. We show that the method recapitulates previous experimental results on immunogenicity reduction, and we use it to disrupt T-cell epitopes in GFP and Pseudomonas exotoxin A without disrupting function.
Keywords: Rosetta; biotherapeutics; deimmunization; immunotoxin; machine learning.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Recombinant immunotoxin for cancer treatment with low immunogenicity by identification and silencing of human T-cell epitopes.Proc Natl Acad Sci U S A. 2014 Jun 10;111(23):8571-6. doi: 10.1073/pnas.1405153111. Epub 2014 May 5. Proc Natl Acad Sci U S A. 2014. PMID: 24799704 Free PMC article.
-
Immunogenicity of therapeutic recombinant immunotoxins.Immunol Rev. 2016 Mar;270(1):152-64. doi: 10.1111/imr.12390. Immunol Rev. 2016. PMID: 26864110 Free PMC article. Review.
-
A guide to taming a toxin--recombinant immunotoxins constructed from Pseudomonas exotoxin A for the treatment of cancer.FEBS J. 2011 Dec;278(23):4683-700. doi: 10.1111/j.1742-4658.2011.08182.x. Epub 2011 Jun 2. FEBS J. 2011. PMID: 21585657 Free PMC article. Review.
-
Higher cytotoxicity of divalent antibody-toxins than monovalent antibody-toxins.Biochem Biophys Res Commun. 2009 Apr 24;382(1):15-20. doi: 10.1016/j.bbrc.2009.02.091. Epub 2009 Feb 24. Biochem Biophys Res Commun. 2009. PMID: 19245794
-
Influence of structural variations on biological activity of anti-PSMA scFv and immunotoxins targeting prostate cancer.Anticancer Res. 2010 Sep;30(9):3373-9. Anticancer Res. 2010. PMID: 20944111
Cited by
-
PITHA: A Webtool to Predict Immunogenicity for Humanized and Fully Human Therapeutic Antibodies.Methods Mol Biol. 2023;2552:143-150. doi: 10.1007/978-1-0716-2609-2_7. Methods Mol Biol. 2023. PMID: 36346590
-
Antibody humanization by structure-based computational protein design.MAbs. 2015;7(6):1045-57. doi: 10.1080/19420862.2015.1076600. Epub 2015 Aug 7. MAbs. 2015. PMID: 26252731 Free PMC article.
-
Computationally optimized deimmunization libraries yield highly mutated enzymes with low immunogenicity and enhanced activity.Proc Natl Acad Sci U S A. 2017 Jun 27;114(26):E5085-E5093. doi: 10.1073/pnas.1621233114. Epub 2017 Jun 12. Proc Natl Acad Sci U S A. 2017. PMID: 28607051 Free PMC article.
-
A review and outlook on expression of animal proteins in plants.Front Plant Sci. 2024 Aug 22;15:1426239. doi: 10.3389/fpls.2024.1426239. eCollection 2024. Front Plant Sci. 2024. PMID: 39239203 Free PMC article. Review.
-
A mathematical model simulating the adaptive immune response in various vaccines and vaccination strategies.Sci Rep. 2024 Oct 14;14(1):23995. doi: 10.1038/s41598-024-74221-x. Sci Rep. 2024. PMID: 39402093 Free PMC article.
References
-
- Zubler RH. Naive and Memory B Cells in T-Cell-Dependent and T-Independent Responses. Springer Seminars in Immunopathology. Berlin: Springer; 2001. pp. 405–419. - PubMed
-
- Goldenberg MM. Trastuzumab, a recombinant DNA-derived humanized monoclonal antibody, a novel agent for the treatment of metastatic breast cancer. Clin Ther. 1999;21(2):309–318. - PubMed
-
- Harding FA, et al. A beta-lactamase with reduced immunogenicity for the targeted delivery of chemotherapeutics using antibody-directed enzyme prodrug therapy. Mol Cancer Ther. 2005;4(11):1791–1800. - PubMed
-
- Tangri S, et al. Rationally engineered therapeutic proteins with reduced immunogenicity. J Immunol. 2005;174(6):3187–3196. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

