Neutrophil extracellular trap-derived enzymes oxidize high-density lipoprotein: an additional proatherogenic mechanism in systemic lupus erythematosus
- PMID: 24838349
- PMCID: PMC4146708
- DOI: 10.1002/art.38703
Neutrophil extracellular trap-derived enzymes oxidize high-density lipoprotein: an additional proatherogenic mechanism in systemic lupus erythematosus
Abstract
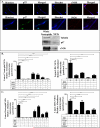
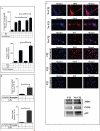
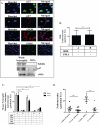
Objective: Oxidative stress and oxidized high-density lipoprotein (HDL) are implicated as risk factors for cardiovascular disease (CVD) in systemic lupus erythematosus (SLE). Yet, how HDL is oxidized and rendered dysfunctional in SLE remains unclear. Neutrophil extracellular traps (NETs), the levels of which are elevated in lupus, possess oxidant-generating enzymes, including myeloperoxidase (MPO), NADPH oxidase (NOX), and nitric oxide synthase (NOS). We hypothesized that NETs mediate HDL oxidation, impairing cholesterol efflux capacity (CEC).
Methods: Plasma MPO levels and CEC activity were examined in controls and lupus patients, and 3-chlorotyrosine (MPO specific) and 3-nitrotyrosine (derived from reactive nitrogen species) were quantified in human HDL. Multivariable linear models were used to estimate and test differences between groups. HDL was exposed to NETs from control and lupus neutrophils in the presence or absence of MPO, NOX, NOS inhibitors, and chloroquine (CQ). Murine HDL oxidation was quantified after NET inhibition in vivo.
Results: SLE patients displayed higher MPO levels and diminished CEC compared to controls. SLE HDL had higher 3-nitrotyrosine and 3-chlorotyrosine content than control HDL, with site-specific oxidation signatures on apolipoprotein A-I. Experiments with human and murine NETs confirmed that chlorination was mediated by MPO and NOX, and nitration by NOS and NOX. Mice with lupus treated with the NET inhibitor Cl-amidine displayed significantly decreased HDL oxidation. CQ inhibited NET formation in vitro.
Conclusion: Active NOS, NOX, and MPO within NETs significantly modify HDL, rendering the lipoprotein proatherogenic. Since NET formation is enhanced in SLE, these findings support a novel role for NET-derived lipoprotein oxidation in SLE-associated CVD and identify additional proatherogenic roles of neutrophils and putative protective roles of antimalarials in autoimmunity.
Published 2014. This article is a U.S. Government work and is in the public domain in the USA.
Figures

Similar articles
-
High density lipoprotein is targeted for oxidation by myeloperoxidase in rheumatoid arthritis.Ann Rheum Dis. 2013 Oct;72(10):1725-31. doi: 10.1136/annrheumdis-2012-202033. Epub 2013 Jan 12. Ann Rheum Dis. 2013. PMID: 23313808 Free PMC article.
-
Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease.J Clin Invest. 2004 Aug;114(4):529-41. doi: 10.1172/JCI21109. J Clin Invest. 2004. PMID: 15314690 Free PMC article.
-
Myeloperoxidase mediated HDL oxidation and HDL proteome changes do not contribute to dysfunctional HDL in Chinese subjects with coronary artery disease.PLoS One. 2018 Mar 5;13(3):e0193782. doi: 10.1371/journal.pone.0193782. eCollection 2018. PLoS One. 2018. PMID: 29505607 Free PMC article.
-
Myeloperoxidase: an oxidative pathway for generating dysfunctional high-density lipoprotein.Chem Res Toxicol. 2010 Mar 15;23(3):447-54. doi: 10.1021/tx9003775. Chem Res Toxicol. 2010. PMID: 20043647 Free PMC article. Review.
-
Myeloperoxidase-mediated oxidation of high-density lipoproteins: fingerprints of newly recognized potential proatherogenic lipoproteins.Arch Biochem Biophys. 2006 Jan 15;445(2):245-55. doi: 10.1016/j.abb.2005.08.008. Epub 2005 Aug 31. Arch Biochem Biophys. 2006. PMID: 16171772 Review.
Cited by
-
Autoimmune diseases and atherosclerotic cardiovascular disease.Nat Rev Cardiol. 2024 Nov;21(11):780-807. doi: 10.1038/s41569-024-01045-7. Epub 2024 Jun 27. Nat Rev Cardiol. 2024. PMID: 38937626 Review.
-
Platelet factor 4 limits neutrophil extracellular trap- and cell-free DNA-induced thrombogenicity and endothelial injury.JCI Insight. 2023 Nov 22;8(22):e171054. doi: 10.1172/jci.insight.171054. JCI Insight. 2023. PMID: 37991024 Free PMC article.
-
Neutrophils-Important Communicators in Systemic Lupus Erythematosus and Antiphospholipid Syndrome.Front Immunol. 2019 Nov 22;10:2734. doi: 10.3389/fimmu.2019.02734. eCollection 2019. Front Immunol. 2019. PMID: 31824510 Free PMC article. Review.
-
Platelet-derived extracellular vesicles released after trauma promote hemostasis and contribute to DVT in mice.J Thromb Haemost. 2019 Oct;17(10):1733-1745. doi: 10.1111/jth.14563. Epub 2019 Jul 28. J Thromb Haemost. 2019. PMID: 31294514 Free PMC article.
-
The role of neutrophils in rheumatic disease-associated vascular inflammation.Nat Rev Rheumatol. 2022 Mar;18(3):158-170. doi: 10.1038/s41584-021-00738-4. Epub 2022 Jan 17. Nat Rev Rheumatol. 2022. PMID: 35039664 Review.
References
-
- Esdaile JM, Abrahamowicz M, Grodzicky T, Li Y, Panaritis C, du Berger R, et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2001;44(10):2331–7. - PubMed
-
- Ronda N, Favari E, Borghi MO, Ingegnoli F, Gerosa M, Chighizola C, et al. Impaired serum cholesterol efflux capacity in rheumatoid arthritis and systemic lupus erythematosus. Ann Rheum Dis. 2014;73(3):609–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL112625/HL/NHLBI NIH HHS/United States
- DK-089503/DK/NIDDK NIH HHS/United States
- R01 HL088419/HL/NHLBI NIH HHS/United States
- R01 GM079357/GM/NIGMS NIH HHS/United States
- GM-079357/GM/NIGMS NIH HHS/United States
- P30 DK020572/DK/NIDDK NIH HHS/United States
- U24 DK097153/DK/NIDDK NIH HHS/United States
- T32 AI007413/AI/NIAID NIH HHS/United States
- DK-097153/DK/NIDDK NIH HHS/United States
- P30 DK017047/DK/NIDDK NIH HHS/United States
- Z99 AR999999/Intramural NIH HHS/United States
- P30 DK089503/DK/NIDDK NIH HHS/United States
- AI-007413/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

