A novel ligand-independent peptide inhibitor of TREM-1 suppresses tumor growth in human lung cancer xenografts and prolongs survival of mice with lipopolysaccharide-induced septic shock
- PMID: 24836682
- PMCID: PMC4088342
- DOI: 10.1016/j.intimp.2014.05.001
A novel ligand-independent peptide inhibitor of TREM-1 suppresses tumor growth in human lung cancer xenografts and prolongs survival of mice with lipopolysaccharide-induced septic shock
Abstract
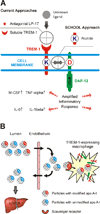
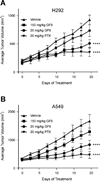
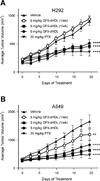
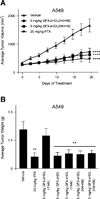
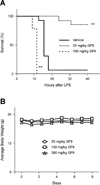
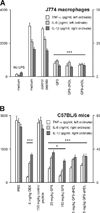
Triggering receptor expressed on myeloid cells-1 (TREM-1) amplifies the inflammatory response and plays a role in cancer and sepsis. Inhibition of TREM-1 by short hairpin RNA (shRNA) in macrophages suppresses cancer cell invasion in vitro. In the clinical setting, high levels of TREM-1 expression on tumor-associated macrophages are associated with cancer recurrence and poor survival of patients with non-small cell lung cancer (NSCLC). TREM-1 upregulation on peritoneal neutrophils has been found in human sepsis patients and in mice with experimental lipopolysaccharide (LPS)-induced septic shock. However, the precise function of TREM-1 and the nature of its ligand are not yet known. In this study, we used the signaling chain homooligomerization (SCHOOL) model of immune signaling to design a novel, ligand-independent peptide-based TREM-1 inhibitor and demonstrated that this peptide specifically silences TREM-1 signaling in vitro and in vivo. Utilizing two human lung tumor xenograft nude mouse models (H292 and A549) and mice with LPS-induced sepsis, we show for the first time that blockade of TREM-1 function using non-toxic and non-immunogenic SCHOOL peptide inhibitors: 1) delays tumor growth in xenograft models of human NSCLC, 2) prolongs survival of mice with LPS-induced septic shock, and 3) substantially decreases cytokine production in vitro and in vivo. In addition, targeted delivery of SCHOOL peptides to macrophages utilizing lipoprotein-mimicking nanoparticles significantly increased peptide half-life and dosage efficacy. Together, the results suggest that ligand-independent modulation of TREM-1 function using small synthetic peptides might be a suitable treatment for sepsis and NSCLC and possibly other types of inflammation-associated disorders.
Keywords: HDL nanoparticles; Non-small cell lung cancer; Sepsis; TREM-1 receptor; Targeted delivery; Therapeutic SCHOOL peptides.
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures



Similar articles
-
Novel TREM-1 Inhibitors Attenuate Tumor Growth and Prolong Survival in Experimental Pancreatic Cancer.Mol Pharm. 2017 Dec 4;14(12):4572-4582. doi: 10.1021/acs.molpharmaceut.7b00711. Epub 2017 Nov 13. Mol Pharm. 2017. PMID: 29095622 Free PMC article.
-
Inhibition of TREM-2 Markedly Suppresses Joint Inflammation and Damage in Experimental Arthritis.Int J Mol Sci. 2022 Aug 9;23(16):8857. doi: 10.3390/ijms23168857. Int J Mol Sci. 2022. PMID: 36012120 Free PMC article.
-
Modulation of the triggering receptor expressed on the myeloid cell type 1 pathway in murine septic shock.Infect Immun. 2006 May;74(5):2823-30. doi: 10.1128/IAI.74.5.2823-2830.2006. Infect Immun. 2006. PMID: 16622220 Free PMC article.
-
Triggering Receptor Expressed on Myeloid Cells (TREM) family and the application of its antagonists.Recent Pat Antiinfect Drug Discov. 2009 Jan;4(1):51-6. doi: 10.2174/157489109787236292. Recent Pat Antiinfect Drug Discov. 2009. PMID: 19149696 Review.
-
TREM-1 Modulation Strategies for Sepsis.Front Immunol. 2022 Jun 15;13:907387. doi: 10.3389/fimmu.2022.907387. eCollection 2022. Front Immunol. 2022. PMID: 35784361 Free PMC article. Review.
Cited by
-
Blocking triggering receptor expressed on myeloid cells-1 attenuates lipopolysaccharide-induced acute lung injury via inhibiting NLRP3 inflammasome activation.Sci Rep. 2016 Dec 22;6:39473. doi: 10.1038/srep39473. Sci Rep. 2016. PMID: 28004759 Free PMC article.
-
Novel ligands and modulators of triggering receptor expressed on myeloid cells receptor family: 2015-2020 updates.Expert Opin Ther Pat. 2021 Jun;31(6):549-561. doi: 10.1080/13543776.2021.1883587. Epub 2021 Feb 8. Expert Opin Ther Pat. 2021. PMID: 33507843 Free PMC article. Review.
-
The role of triggering receptor expressed on myeloid cells-1 (TREM-1) in central nervous system diseases.Mol Brain. 2022 Oct 22;15(1):84. doi: 10.1186/s13041-022-00969-w. Mol Brain. 2022. PMID: 36273145 Free PMC article. Review.
-
Pharmacological Inhibition or Silencing of TREM1 Restrains HCC Cell Metastasis by Inactivating TLR/PI3K/AKT Signaling.Cell Biochem Biophys. 2024 Sep;82(3):2673-2685. doi: 10.1007/s12013-024-01377-8. Epub 2024 Jul 2. Cell Biochem Biophys. 2024. PMID: 38954352
-
Bulk and Single-Cell Profiling of Breast Tumors Identifies TREM-1 as a Dominant Immune Suppressive Marker Associated With Poor Outcomes.Front Oncol. 2021 Dec 8;11:734959. doi: 10.3389/fonc.2021.734959. eCollection 2021. Front Oncol. 2021. PMID: 34956864 Free PMC article.
References
-
- Felip E, Santarpia M, Rosell R. Emerging drugs for non-small-cell lung cancer. Expert Opin Emerg Drugs. 2007;12:449–460. - PubMed
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. - PubMed
-
- Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86:1065–1073. - PubMed
-
- Shih J-Y, Yuan A, Chen JJ-W, Yang P-C. Tumor-Associated Macrophage: Its Role in Cancer Invasion and Metastasis. J Cancer Mol. 2006;2:101–106.
-
- Welsh TJ, Green RH, Richardson D, Waller DA, O'Byrne KJ, Bradding P. Macrophage and mast-cell invasion of tumor cell islets confers a marked survival advantage in non-small-cell lung cancer. J Clin Oncol. 2005;23:8959–8967. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

