The role of the cell wall in plant immunity
- PMID: 24834069
- PMCID: PMC4018530
- DOI: 10.3389/fpls.2014.00178
The role of the cell wall in plant immunity
Abstract
The battle between plants and microbes is evolutionarily ancient, highly complex, and often co-dependent. A primary challenge for microbes is to breach the physical barrier of host cell walls whilst avoiding detection by the plant's immune receptors. While some receptors sense conserved microbial features, others monitor physical changes caused by an infection attempt. Detection of microbes leads to activation of appropriate defense responses that then challenge the attack. Plant cell walls are formidable and dynamic barriers. They are constructed primarily of complex carbohydrates joined by numerous distinct connection types, and are subject to extensive post-synthetic modification to suit prevailing local requirements. Multiple changes can be triggered in cell walls in response to microbial attack. Some of these are well described, but many remain obscure. The study of the myriad of subtle processes underlying cell wall modification poses special challenges for plant glycobiology. In this review we describe the major molecular and cellular mechanisms that underlie the roles of cell walls in plant defense against pathogen attack. In so doing, we also highlight some of the challenges inherent in studying these interactions, and briefly describe the analytical potential of molecular probes used in conjunction with carbohydrate microarray technology.
Keywords: DAMP; PAMP; PTI; callose; chitin; defense; immunity; plant cell wall.
Figures
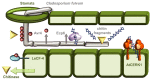
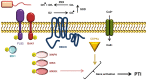
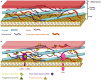
Similar articles
-
Glycans as Modulators of Plant Defense Against Filamentous Pathogens.Front Plant Sci. 2018 Jul 4;9:928. doi: 10.3389/fpls.2018.00928. eCollection 2018. Front Plant Sci. 2018. PMID: 30022987 Free PMC article. Review.
-
The battle for chitin recognition in plant-microbe interactions.FEMS Microbiol Rev. 2015 Mar;39(2):171-83. doi: 10.1093/femsre/fuu003. Epub 2014 Dec 22. FEMS Microbiol Rev. 2015. PMID: 25725011 Review.
-
The plant cell wall: a dynamic barrier against pathogen invasion.Front Plant Sci. 2012 May 7;3:85. doi: 10.3389/fpls.2012.00085. eCollection 2012. Front Plant Sci. 2012. PMID: 22639669 Free PMC article.
-
Arabidopsis Plasma Membrane ATPase AHA5 Is Negatively Involved in PAMP-Triggered Immunity.Int J Mol Sci. 2022 Mar 31;23(7):3857. doi: 10.3390/ijms23073857. Int J Mol Sci. 2022. PMID: 35409217 Free PMC article.
-
Role of RIN4 in Regulating PAMP-Triggered Immunity and Effector-Triggered Immunity: Current Status and Future Perspectives.Mol Cells. 2019 Jul 31;42(7):503-511. doi: 10.14348/molcells.2019.2433. Mol Cells. 2019. PMID: 31362467 Free PMC article. Review.
Cited by
-
The Resistant Soybean-Aphis glycines Interaction: Current Knowledge and Prospects.Front Plant Sci. 2020 Aug 11;11:1223. doi: 10.3389/fpls.2020.01223. eCollection 2020. Front Plant Sci. 2020. PMID: 32849757 Free PMC article. Review.
-
The Plant Cell Wall: A Complex and Dynamic Structure As Revealed by the Responses of Genes under Stress Conditions.Front Plant Sci. 2016 Aug 10;7:984. doi: 10.3389/fpls.2016.00984. eCollection 2016. Front Plant Sci. 2016. PMID: 27559336 Free PMC article. Review.
-
Transcriptome analysis provides insights into the bases of salicylic acid-induced resistance to anthracnose in sorghum.Plant Mol Biol. 2022 Sep;110(1-2):69-80. doi: 10.1007/s11103-022-01286-5. Epub 2022 Jul 6. Plant Mol Biol. 2022. PMID: 35793006
-
Role of Protein Glycosylation in Host-Pathogen Interaction.Cells. 2020 Apr 20;9(4):1022. doi: 10.3390/cells9041022. Cells. 2020. PMID: 32326128 Free PMC article. Review.
-
Oxathiapiprolin, a Novel Chemical Inducer Activates the Plant Disease Resistance.Int J Mol Sci. 2020 Feb 12;21(4):1223. doi: 10.3390/ijms21041223. Int J Mol Sci. 2020. PMID: 32059380 Free PMC article.
References
-
- Albersheim P., Darvill A., Roberts K., Sederoff R., Staehelin A. (ed.). (2011). Plant Cell Walls. New York, NY: Garland Science, Taylor and Francis Publishing Group, LLC; 52–61
-
- Annis S. L., Goodwin P. H. (1997). Recent advances in the molecular genetics of plant cell wall-degrading enzymes produced by plant pathogenic fungi. Eur. J. Plant Pathol. 103 1–14 10.1023/A:1008656013255 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

