Interactions between exosomes from breast cancer cells and primary mammary epithelial cells leads to generation of reactive oxygen species which induce DNA damage response, stabilization of p53 and autophagy in epithelial cells
- PMID: 24831807
- PMCID: PMC4022578
- DOI: 10.1371/journal.pone.0097580
Interactions between exosomes from breast cancer cells and primary mammary epithelial cells leads to generation of reactive oxygen species which induce DNA damage response, stabilization of p53 and autophagy in epithelial cells
Abstract
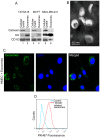
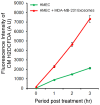
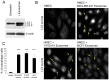
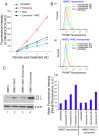
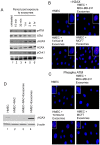
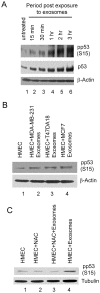
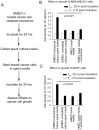
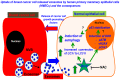
Exosomes are nanovesicles originating from multivesicular bodies and are released by all cell types. They contain proteins, lipids, microRNAs, mRNAs and DNA fragments, which act as mediators of intercellular communications by inducing phenotypic changes in recipient cells. Tumor-derived exosomes have been shown to play critical roles in different stages of tumor development and metastasis of almost all types of cancer. One of the ways by which exosomes affect tumorigenesis is to manipulate the tumor microenvironments to create tumor permissive "niches". Whether breast cancer cell secreted exosomes manipulate epithelial cells of the mammary duct to facilitate tumor development is not known. To address whether and how breast cancer cell secreted exosomes manipulate ductal epithelial cells we studied the interactions between exosomes isolated from conditioned media of 3 different breast cancer cell lines (MDA-MB-231, T47DA18 and MCF7), representing three different types of breast carcinomas, and normal human primary mammary epithelial cells (HMECs). Our studies show that exosomes released by breast cancer cell lines are taken up by HMECs, resulting in the induction of reactive oxygen species (ROS) and autophagy. Inhibition of ROS by N-acetyl-L-cysteine (NAC) led to abrogation of autophagy. HMEC-exosome interactions also induced the phosphorylation of ATM, H2AX and Chk1 indicating the induction of DNA damage repair (DDR) responses. Under these conditions, phosphorylation of p53 at serine 15 was also observed. Both DDR responses and phosphorylation of p53 induced by HMEC-exosome interactions were also inhibited by NAC. Furthermore, exosome induced autophagic HMECs were found to release breast cancer cell growth promoting factors. Taken together, our results suggest novel mechanisms by which breast cancer cell secreted exosomes manipulate HMECs to create a tumor permissive microenvironment.
Conflict of interest statement
Figures
Similar articles
-
Psoralidin induced reactive oxygen species (ROS)-dependent DNA damage and protective autophagy mediated by NOX4 in breast cancer cells.Phytomedicine. 2016 Aug 15;23(9):939-47. doi: 10.1016/j.phymed.2016.05.008. Epub 2016 May 24. Phytomedicine. 2016. PMID: 27387402
-
Secretion modification region-derived peptide blocks exosome release and mediates cell cycle arrest in breast cancer cells.Oncotarget. 2017 Feb 14;8(7):11302-11315. doi: 10.18632/oncotarget.14513. Oncotarget. 2017. PMID: 28076321 Free PMC article.
-
Role of p53 and reactive oxygen species in apoptotic response to copper and zinc in epithelial breast cancer cells.Apoptosis. 2005 Jan;10(1):111-21. doi: 10.1007/s10495-005-6066-7. Apoptosis. 2005. PMID: 15711927
-
Exosome: emerging biomarker in breast cancer.Oncotarget. 2017 Jun 20;8(25):41717-41733. doi: 10.18632/oncotarget.16684. Oncotarget. 2017. PMID: 28402944 Free PMC article. Review.
-
Cancer cell's internal and external warriors: Autophagosomes and exosomes.Life Sci. 2022 Jul 1;300:120552. doi: 10.1016/j.lfs.2022.120552. Epub 2022 Apr 19. Life Sci. 2022. PMID: 35452638 Review.
Cited by
-
Extracellular Vesicles in Modifying the Effects of Ionizing Radiation.Int J Mol Sci. 2019 Nov 6;20(22):5527. doi: 10.3390/ijms20225527. Int J Mol Sci. 2019. PMID: 31698689 Free PMC article. Review.
-
Exosomes derived from HeLa cells break down vascular integrity by triggering endoplasmic reticulum stress in endothelial cells.J Extracell Vesicles. 2020 Feb 3;9(1):1722385. doi: 10.1080/20013078.2020.1722385. eCollection 2020. J Extracell Vesicles. 2020. PMID: 32128072 Free PMC article.
-
ATM at the crossroads of reactive oxygen species and autophagy.Int J Biol Sci. 2021 Jul 22;17(12):3080-3090. doi: 10.7150/ijbs.63963. eCollection 2021. Int J Biol Sci. 2021. PMID: 34421351 Free PMC article. Review.
-
Tumor-derived exosomes in cancer progression and treatment failure.Oncotarget. 2015 Nov 10;6(35):37151-68. doi: 10.18632/oncotarget.6022. Oncotarget. 2015. PMID: 26452221 Free PMC article. Review.
-
Exosomal cargos modulate autophagy in recipient cells via different signaling pathways.Cell Biosci. 2020 Aug 1;10:92. doi: 10.1186/s13578-020-00455-7. eCollection 2020. Cell Biosci. 2020. PMID: 32765827 Free PMC article. Review.
References
-
- Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, et al... (eds) (2012) SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations). National Cancer Institute. Bethesda, MD, 2012.
-
- Leonard GD, Swain SM (2004) Ductal carcinoma in situ, complexities and challenges. J Natl Cancer Inst 96: 906–920. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

