The downregulation of GFI1 by the EZH2-NDY1/KDM2B-JARID2 axis and by human cytomegalovirus (HCMV) associated factors allows the activation of the HCMV major IE promoter and the transition to productive infection
- PMID: 24830456
- PMCID: PMC4022736
- DOI: 10.1371/journal.ppat.1004136
The downregulation of GFI1 by the EZH2-NDY1/KDM2B-JARID2 axis and by human cytomegalovirus (HCMV) associated factors allows the activation of the HCMV major IE promoter and the transition to productive infection
Abstract
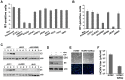
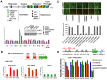
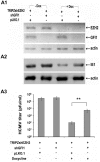
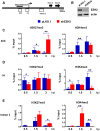
Earlier studies had suggested that epigenetic mechanisms play an important role in the control of human cytomegalovirus (HCMV) infection. Here we show that productive HCMV infection is indeed under the control of histone H3K27 trimethylation. The histone H3K27 methyltransferase EZH2, and its regulators JARID2 and NDY1/KDM2B repress GFI1, a transcriptional repressor of the major immediate-early promoter (MIEP) of HCMV. Knocking down EZH2, NDY1/KDM2B or JARID2 relieves the repression and results in the upregulation of GFI1. During infection, the incoming HCMV rapidly downregulates the GFI1 mRNA and protein in both wild-type cells and in cells in which EZH2, NDY1/KDM2B or JARID2 were knocked down. However, since the pre-infection levels of GFI1 in the latter cells are significantly higher, the virus fails to downregulate it to levels permissive for MIEP activation and viral infection. Following the EZH2-NDY1/KDM2B-JARID2-independent downregulation of GFI1 in the early stages of infection, the virus also initiates an EZH2-NDY1/ΚDM2Β-JARID2-dependent program that represses GFI1 throughout the infection cycle. The EZH2 knockdown also delays histone H3K27 trimethylation in the immediate early region of HCMV, which is accompanied by a drop in H3K4 trimethylation that may contribute to the shEZH2-mediated repression of the major immediate early HCMV promoter. These data show that HCMV uses multiple mechanisms to allow the activation of the HCMV MIEP and to prevent cellular mechanisms from blocking the HCMV replication program.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
FGF-2 regulates cell proliferation, migration, and angiogenesis through an NDY1/KDM2B-miR-101-EZH2 pathway.Mol Cell. 2011 Jul 22;43(2):285-98. doi: 10.1016/j.molcel.2011.06.020. Mol Cell. 2011. PMID: 21777817 Free PMC article.
-
Chromatin-Remodeling Factor SPOC1 Acts as a Cellular Restriction Factor against Human Cytomegalovirus by Repressing the Major Immediate Early Promoter.J Virol. 2018 Jun 29;92(14):e00342-18. doi: 10.1128/JVI.00342-18. Print 2018 Jul 15. J Virol. 2018. PMID: 29743358 Free PMC article.
-
A Noncanonical Function of Polycomb Repressive Complexes Promotes Human Cytomegalovirus Lytic DNA Replication and Serves as a Novel Cellular Target for Antiviral Intervention.J Virol. 2019 Apr 17;93(9):e02143-18. doi: 10.1128/JVI.02143-18. Print 2019 May 1. J Virol. 2019. PMID: 30814291 Free PMC article.
-
Chromatin-mediated regulation of cytomegalovirus gene expression.Virus Res. 2011 May;157(2):134-43. doi: 10.1016/j.virusres.2010.09.019. Epub 2010 Sep 25. Virus Res. 2011. PMID: 20875471 Free PMC article. Review.
-
Chromatin structure regulates human cytomegalovirus gene expression during latency, reactivation and lytic infection.Biochim Biophys Acta. 2010 Mar-Apr;1799(3-4):286-95. doi: 10.1016/j.bbagrm.2009.08.001. Epub 2009 Aug 12. Biochim Biophys Acta. 2010. PMID: 19682613 Review.
Cited by
-
Histone H3K14 hypoacetylation and H3K27 hypermethylation along with HDAC1 up-regulation and KDM6B down-regulation are associated with active pulmonary tuberculosis disease.Am J Transl Res. 2017 Apr 15;9(4):1943-1955. eCollection 2017. Am J Transl Res. 2017. PMID: 28469799 Free PMC article.
-
Expanding the Known Functional Repertoire of the Human Cytomegalovirus pp71 Protein.Front Cell Infect Microbiol. 2020 Mar 12;10:95. doi: 10.3389/fcimb.2020.00095. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32226778 Free PMC article. Review.
-
Chromatin control of human cytomegalovirus infection.mBio. 2023 Aug 31;14(4):e0032623. doi: 10.1128/mbio.00326-23. Epub 2023 Jul 13. mBio. 2023. PMID: 37439556 Free PMC article. Review.
-
Cytomegalovirus Latency and Reactivation: An Intricate Interplay With the Host Immune Response.Front Cell Infect Microbiol. 2020 Mar 31;10:130. doi: 10.3389/fcimb.2020.00130. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32296651 Free PMC article. Review.
-
Analyses of publicly available genomics resources define FGF-2-expressing bladder carcinomas as EMT-prone, proliferative tumors with low mutation rates and high expression of CTLA-4, PD-1 and PD-L1.Signal Transduct Target Ther. 2017;2:16045-. doi: 10.1038/sigtrans.2016.45. Epub 2017 Mar 17. Signal Transduct Target Ther. 2017. PMID: 28515962 Free PMC article.
References
-
- Sinzger C, Grefte A, Plachter B, Gouw AS, The TH, et al. (1995) Fibroblasts, epithelial cells, endothelial cells and smooth muscle cells are major targets of human cytomegalovirus infection in lung and gastrointestinal tissues. J Gen Virol 76 (Pt 4): 741–750. - PubMed
-
- Sinclair J (2008) Human cytomegalovirus: Latency and reactivation in the myeloid lineage. J Clin Virol 41: 180–185. - PubMed
-
- Streblow DN, Nelson JA (2003) Models of HCMV latency and reactivation. Trends Microbiol 11: 293–295. - PubMed
-
- Castillo JP, Kowalik TF (2002) Human cytomegalovirus immediate early proteins and cell growth control. Gene 290: 19–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

