Mutation in VPS35 associated with Parkinson's disease impairs WASH complex association and inhibits autophagy
- PMID: 24819384
- PMCID: PMC4024763
- DOI: 10.1038/ncomms4828
Mutation in VPS35 associated with Parkinson's disease impairs WASH complex association and inhibits autophagy
Abstract
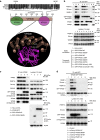
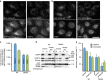
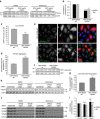
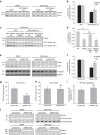
Endosomal protein sorting controls the localization of many physiologically important proteins and is linked to several neurodegenerative diseases. VPS35 is a component of the retromer complex, which mediates endosome-to-Golgi retrieval of membrane proteins such as the cation-independent mannose 6-phosphate receptor. Furthermore, retromer is also required for the endosomal recruitment of the actin nucleation promoting WASH complex. The VPS35 D620N mutation causes a rare form of autosomal-dominant Parkinson's disease (PD). Here we show that this mutant associates poorly with the WASH complex and impairs WASH recruitment to endosomes. Autophagy is impaired in cells expressing PD-mutant VPS35 or lacking WASH. The autophagy defects can be explained, at least in part, by abnormal trafficking of the autophagy protein ATG9A. Thus, the PD-causing D620N mutation in VPS35 restricts WASH complex recruitment to endosomes, and reveals a novel role for the WASH complex in autophagosome formation.
Figures

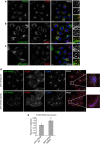
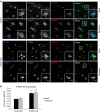
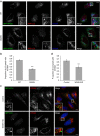
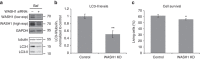
Comment in
-
VPS35 Parkinson mutation impairs autophagy via WASH.Cell Cycle. 2014;13(14):2155-6. doi: 10.4161/cc.29734. Epub 2014 Jun 25. Cell Cycle. 2014. PMID: 24963965 Free PMC article. No abstract available.
Similar articles
-
Retromer binding to FAM21 and the WASH complex is perturbed by the Parkinson disease-linked VPS35(D620N) mutation.Curr Biol. 2014 Jul 21;24(14):1670-1676. doi: 10.1016/j.cub.2014.06.024. Epub 2014 Jul 3. Curr Biol. 2014. PMID: 24980502 Free PMC article.
-
Formation of retromer transport carriers is disrupted by the Parkinson disease-linked Vps35 D620N variant.Traffic. 2021 Apr;22(4):123-136. doi: 10.1111/tra.12779. Epub 2021 Jan 22. Traffic. 2021. PMID: 33347683
-
The Vps35 D620N mutation linked to Parkinson's disease disrupts the cargo sorting function of retromer.Traffic. 2014 Feb;15(2):230-44. doi: 10.1111/tra.12136. Epub 2013 Nov 14. Traffic. 2014. PMID: 24152121
-
VPS35 and retromer dysfunction in Parkinson's disease.Philos Trans R Soc Lond B Biol Sci. 2024 Apr 8;379(1899):20220384. doi: 10.1098/rstb.2022.0384. Epub 2024 Feb 19. Philos Trans R Soc Lond B Biol Sci. 2024. PMID: 38368930 Free PMC article. Review.
-
Understanding the contributions of VPS35 and the retromer in neurodegenerative disease.Neurobiol Dis. 2022 Aug;170:105768. doi: 10.1016/j.nbd.2022.105768. Epub 2022 May 16. Neurobiol Dis. 2022. PMID: 35588987 Free PMC article. Review.
Cited by
-
The Role of Vesicle Trafficking Defects in the Pathogenesis of Prion and Prion-Like Disorders.Int J Mol Sci. 2020 Sep 23;21(19):7016. doi: 10.3390/ijms21197016. Int J Mol Sci. 2020. PMID: 32977678 Free PMC article. Review.
-
Neuronal Autophagy: Regulations and Implications in Health and Disease.Cells. 2024 Jan 4;13(1):103. doi: 10.3390/cells13010103. Cells. 2024. PMID: 38201307 Free PMC article. Review.
-
The Parkinson's disease related mutant VPS35 (D620N) amplifies the LRRK2 response to endolysosomal stress.Biochem J. 2024 Feb 21;481(4):265-278. doi: 10.1042/BCJ20230492. Biochem J. 2024. PMID: 38299383 Free PMC article.
-
Lysosomal dysfunction in α-synuclein pathology: molecular mechanisms and therapeutic strategies.Cell Mol Life Sci. 2024 Sep 3;81(1):382. doi: 10.1007/s00018-024-05419-5. Cell Mol Life Sci. 2024. PMID: 39223418 Free PMC article. Review.
-
APEX2-mediated RAB proximity labeling identifies a role for RAB21 in clathrin-independent cargo sorting.EMBO Rep. 2019 Feb;20(2):e47192. doi: 10.15252/embr.201847192. Epub 2019 Jan 3. EMBO Rep. 2019. PMID: 30610016 Free PMC article.
References
-
- Derivery E. et al. The Arp2/3 Activator WASH controls the fission of endosomes through a large multiprotein complex. Dev. Cell 17, 712–723 (2009). - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

