Maintenance treatment with the immunomodulator MGN1703, a Toll-like receptor 9 (TLR9) agonist, in patients with metastatic colorectal carcinoma and disease control after chemotherapy: a randomised, double-blind, placebo-controlled trial
- PMID: 24816725
- PMCID: PMC4131138
- DOI: 10.1007/s00432-014-1682-7
Maintenance treatment with the immunomodulator MGN1703, a Toll-like receptor 9 (TLR9) agonist, in patients with metastatic colorectal carcinoma and disease control after chemotherapy: a randomised, double-blind, placebo-controlled trial
Abstract
Purpose: This phase II study evaluated the synthetic DNA-based immunomodulator and Toll-like receptor 9 agonist MGN1703 as maintenance treatment in metastatic colorectal carcinoma (mCRC).
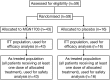
Methods: Fifty-nine patients with mCRC and disease control after standard first-line chemotherapy were randomised to MGN1703 60 mg (N = 43) or placebo (N = 16).
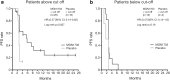
Results: The hazard ratio (HR) for the primary endpoint [progression-free survival (PFS) from the start of maintenance] was 0.56 (95 % CI 0.29-1.08; P = 0.07) and 0.55 (95 % CI 0.3-1.0; P = 0.04) by independent and investigator review, respectively. MGN1703 significantly improved PFS measured from the start of induction therapy versus placebo on independent (HR 0.49; 95 % CI 0.26-0.94; P = 0.03) and investigator review (HR 0.50; 95 % CI 0.31-1.02; P = 0.02). Overall survival (OS) data remain immature (HR 95 %; 95 % CI 0.3-1.5; P = 0.29) with 28/43 patients alive after a medium follow-up of >17 months. Retrospective subgroup analysis showed a significant effect of MGN1703 on PFS versus placebo in patients with greater than median tumour size reduction and normalised carcinoembryonic antigen concentrations following induction therapy, and in patients with elevated activated NKT cells ≥3.08 %. Adverse events were mild to moderate and limited to injection-site reactions or linked to general immune system activation.
Conclusions: MGN1703 maintenance treatment was well tolerated and appears to induce durable and prolonged PFS and disease control in a subgroup of patients with mCRC following induction therapy. Activated NKT cells may be a predictive biomarker for selecting patients likely to benefit more from MGN1703.
Figures

Similar articles
-
Phase I clinical study of the toll-like receptor 9 agonist MGN1703 in patients with metastatic solid tumours.Eur J Cancer. 2015 Jan;51(2):146-56. doi: 10.1016/j.ejca.2014.11.002. Epub 2014 Dec 2. Eur J Cancer. 2015. PMID: 25480557 Clinical Trial.
-
A double-blind, randomized, placebo-controlled, phase 2 study of maintenance enzastaurin with 5-fluorouracil/leucovorin plus bevacizumab after first-line therapy for metastatic colorectal cancer.Cancer. 2012 Sep 1;118(17):4132-8. doi: 10.1002/cncr.26692. Epub 2011 Dec 27. Cancer. 2012. PMID: 22213153 Clinical Trial.
-
Subgroup analysis in RAISE: a randomized, double-blind phase III study of irinotecan, folinic acid, and 5-fluorouracil (FOLFIRI) plus ramucirumab or placebo in patients with metastatic colorectal carcinoma progression.Ann Oncol. 2016 Nov;27(11):2082-2090. doi: 10.1093/annonc/mdw402. Epub 2016 Aug 29. Ann Oncol. 2016. PMID: 27573561 Free PMC article. Clinical Trial.
-
MGN1703, an immunomodulator and toll-like receptor 9 (TLR-9) agonist: from bench to bedside.Crit Rev Oncol Hematol. 2015 Apr;94(1):31-44. doi: 10.1016/j.critrevonc.2014.12.002. Epub 2014 Dec 19. Crit Rev Oncol Hematol. 2015. PMID: 25577571 Review.
-
Poly(ADP-ribose) polymerase (PARP) inhibitors for the treatment of ovarian cancer.Cochrane Database Syst Rev. 2022 Feb 16;2(2):CD007929. doi: 10.1002/14651858.CD007929.pub4. Cochrane Database Syst Rev. 2022. PMID: 35170751 Free PMC article. Review.
Cited by
-
Trial Watch: Immunostimulation with Toll-like receptor agonists in cancer therapy.Oncoimmunology. 2015 Sep 2;5(3):e1088631. doi: 10.1080/2162402X.2015.1088631. eCollection 2016 Mar. Oncoimmunology. 2015. PMID: 27141345 Free PMC article. Review.
-
Engaging Pattern Recognition Receptors in Solid Tumors to Generate Systemic Antitumor Immunity.Cancer Treat Res. 2022;183:91-129. doi: 10.1007/978-3-030-96376-7_3. Cancer Treat Res. 2022. PMID: 35551657
-
Nanoparticle delivery of immunostimulatory oligonucleotides enhances response to checkpoint inhibitor therapeutics.Proc Natl Acad Sci U S A. 2020 Jun 16;117(24):13428-13436. doi: 10.1073/pnas.2001569117. Epub 2020 Jun 3. Proc Natl Acad Sci U S A. 2020. PMID: 32493746 Free PMC article.
-
Advances in immunotherapy for colorectal cancer: a review.Therap Adv Gastroenterol. 2020 Jun 1;13:1756284820917527. doi: 10.1177/1756284820917527. eCollection 2020. Therap Adv Gastroenterol. 2020. PMID: 32536977 Free PMC article. Review.
-
Short-Course Toll-Like Receptor 9 Agonist Treatment Impacts Innate Immunity and Plasma Viremia in Individuals With Human Immunodeficiency Virus Infection.Clin Infect Dis. 2017 Jun 15;64(12):1686-1695. doi: 10.1093/cid/cix201. Clin Infect Dis. 2017. PMID: 28329286 Free PMC article. Clinical Trial.
References
-
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. - DOI - PMC - PubMed
-
- Carpentier A, Laigle-Donadey F, Zohar S, Capelle L, Behin A, Tibi A, Martin-Duverneuil N, Sanson M, Lacomblez L, Taillibert S, Puybasset L, Van Effenterre R, Delattre JY, Carpentier AF. Phase 1 trial of a CpG oligodeoxynucleotide for patients with recurrent glioblastoma. Neuro Oncol. 2006;8:60–66. doi: 10.1215/S1522851705000475. - DOI - PMC - PubMed
-
- Chu E. An update on the current and emerging targeted agents in metastatic colorectal cancer. Clin Colorectal Cancer. 2012;11:1–13. - PubMed
-
- Chung KY, Gore I, Fong L, Venook A, Beck SB, Dorazio P, Criscitiello PJ, Healey DI, Huang B, Gomez-Navarro J, Saltz LB. Phase II study of the anti-cytotoxic T-lymphocyte-associated antigen 4 monoclonal antibody, tremelimumab, in patients with refractory metastatic colorectal cancer. J Clin Oncol. 2010;28:3485–3490. doi: 10.1200/JCO.2010.28.3994. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

