Long-term therapy of chronic delta hepatitis with peginterferon alfa
- PMID: 24815494
- PMCID: PMC5510621
- DOI: 10.1111/apt.12788
Long-term therapy of chronic delta hepatitis with peginterferon alfa
Abstract
Background: Chronic delta hepatitis virus (HDV) infection rapidly progresses to cirrhosis. Treatment with peginterferon for up to 2 years is often without durable response.
Aim: To examine the efficacy and safety of long-term peginterferon in achieving a durable response.
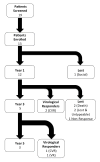
Methods: Treatment was initiated with 180 μg/week of peginterferon alfa-2a with titration to a maximal tolerable dose, for up to 5 years. Liver biopsies and hepatic venous pressure gradients (HVPG) were evaluated at baseline, 1, 3 and 5 years. The primary endpoint was histological improvement or loss of serum HDV and HBsAg at 3 years.
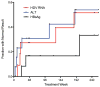
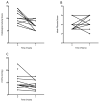
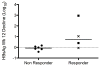
Results: Thirteen patients were treated for a median of 140 weeks (6-260) with an average peginterferon dose of 180 μg/week (90-270). At baseline, most had advanced disease (median Ishak fibrosis = 3) with portal hypertension (HVPG = 10.2 ± 6 mmHg). Five of 13 patients (39%) achieved the primary endpoint, with three seroconverting for HBsAg after 24, 37 and 202 weeks of treatment. Histological inflammation improved after 1 year, (median HAI: 10 vs. 7, P = 0.01) with persistence in 4/5 patients at 3 years (median HAI: 7.5). Greatest improvements occurred in the first year. Baseline bilirubin and HBsAg levels were significantly lower in virological responders than nonresponders. After 12 weeks, virological responders had a significant decline in HBsAg (1.5 log10 IU/mL, P = 0.05).
Conclusion: Despite increased doses and duration of therapy, treatment of chronic HDV with peginterferon remains unsatisfactory. Quantitative measures of HBsAg may be an important biomarker of early response to peginterferon therapy in chronic delta hepatitis virus infection.
Published 2014. This article is a US Government work and is in the public domain in the USA.
Figures

Comment in
-
Letter: the potential success of long-term therapy of chronic delta hepatitis with peginterferon alfa.Aliment Pharmacol Ther. 2015 Mar;41(6):595. doi: 10.1111/apt.13071. Aliment Pharmacol Ther. 2015. PMID: 25659214 No abstract available.
-
Letter: the potential success of long-term therapy of chronic delta hepatitis with peginterferon alfa--authors' reply.Aliment Pharmacol Ther. 2015 Mar;41(6):595-6. doi: 10.1111/apt.13079. Aliment Pharmacol Ther. 2015. PMID: 25659215 No abstract available.
-
Letter: cure of chronic hepatitis B and D after 12 years of treatment with low-dose standard interferon alfa-2b.Aliment Pharmacol Ther. 2016 Sep;44(6):649-50. doi: 10.1111/apt.13705. Aliment Pharmacol Ther. 2016. PMID: 27511138 No abstract available.
Similar articles
-
Peginterferon plus adefovir versus either drug alone for hepatitis delta.N Engl J Med. 2011 Jan 27;364(4):322-31. doi: 10.1056/NEJMoa0912696. N Engl J Med. 2011. PMID: 21268724 Clinical Trial.
-
The efficacy of the Peginterferon treatment in chronic hepatitis HDV and compensate liver cirrhosis.Rev Med Chir Soc Med Nat Iasi. 2014 Apr-Jun;118(2):368-75. Rev Med Chir Soc Med Nat Iasi. 2014. PMID: 25076702
-
Peginterferon alfa-2a plus tenofovir disoproxil fumarate for hepatitis D (HIDIT-II): a randomised, placebo controlled, phase 2 trial.Lancet Infect Dis. 2019 Mar;19(3):275-286. doi: 10.1016/S1473-3099(18)30663-7. Lancet Infect Dis. 2019. PMID: 30833068 Clinical Trial.
-
Current management of delta hepatitis.Liver Int. 2013 Feb;33 Suppl 1:195-7. doi: 10.1111/liv.12058. Liver Int. 2013. PMID: 23286865 Review.
-
Spotlight on peginterferon-alpha-2a (40KD) in chronic hepatitis C.BioDrugs. 2002;16(3):213-7. doi: 10.2165/00063030-200216030-00006. BioDrugs. 2002. PMID: 12102649 Review.
Cited by
-
Present and future management of viral hepatitis.World J Gastroenterol. 2021 Dec 21;27(47):8081-8102. doi: 10.3748/wjg.v27.i47.8081. World J Gastroenterol. 2021. PMID: 35068856 Free PMC article. Review.
-
Hepatitis D virus in 2021: virology, immunology and new treatment approaches for a difficult-to-treat disease.Gut. 2021 Sep;70(9):1782-1794. doi: 10.1136/gutjnl-2020-323888. Epub 2021 Jun 8. Gut. 2021. PMID: 34103404 Free PMC article. Review.
-
Hepatitis delta virus: A fascinating and neglected pathogen.World J Virol. 2015 Nov 12;4(4):313-22. doi: 10.5501/wjv.v4.i4.313. World J Virol. 2015. PMID: 26568914 Free PMC article. Review.
-
Therapeutic Advances in Viral Hepatitis A-E.Adv Ther. 2022 Apr;39(4):1524-1552. doi: 10.1007/s12325-022-02070-z. Epub 2022 Feb 27. Adv Ther. 2022. PMID: 35220557 Review.
-
Hepatitis D infection: from initial discovery to current investigational therapies.Gastroenterol Rep (Oxf). 2019 Jun 23;7(4):231-245. doi: 10.1093/gastro/goz023. eCollection 2019 Aug. Gastroenterol Rep (Oxf). 2019. PMID: 32477569 Free PMC article.
References
-
- Hughes SA, Wedemeyer H, Harrison PM. Hepatitis delta virus. Lancet. 2011;378:73–85. - PubMed
-
- Rizzetto M. Hepatitis D: thirty years after. Journal of hepatology. 2009;50:1043–50. - PubMed
-
- Rizzetto M, Ponzetto A, Forzani I. Hepatitis delta virus as a global health problem. Vaccine. 1990;8(Suppl):S10–4. discussion S21–3. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
