Leptin-dependent neuronal NO signaling in the preoptic hypothalamus facilitates reproduction
- PMID: 24812663
- PMCID: PMC4089460
- DOI: 10.1172/JCI65928
Leptin-dependent neuronal NO signaling in the preoptic hypothalamus facilitates reproduction
Erratum in
- J Clin Invest. 2014 Aug 1;124(8):3678
Abstract
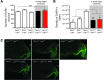
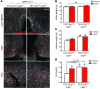
The transition to puberty and adult fertility both require a minimum level of energy availability. The adipocyte-derived hormone leptin signals the long-term status of peripheral energy stores and serves as a key metabolic messenger to the neuroendocrine reproductive axis. Humans and mice lacking leptin or its receptor fail to complete puberty and are infertile. Restoration of leptin levels in these individuals promotes sexual maturation, which requires the pulsatile, coordinated delivery of gonadotropin-releasing hormone to the pituitary and the resulting surge of luteinizing hormone (LH); however, the neural circuits that control the leptin-mediated induction of the reproductive axis are not fully understood. Here, we found that leptin coordinated fertility by acting on neurons in the preoptic region of the hypothalamus and inducing the synthesis of the freely diffusible volume-based transmitter NO, through the activation of neuronal NO synthase (nNOS) in these neurons. The deletion of the gene encoding nNOS or its pharmacological inhibition in the preoptic region blunted the stimulatory action of exogenous leptin on LH secretion and prevented the restoration of fertility in leptin-deficient female mice by leptin treatment. Together, these data indicate that leptin plays a central role in regulating the hypothalamo-pituitary-gonadal axis in vivo through the activation of nNOS in neurons of the preoptic region.
Figures
Similar articles
-
Kisspeptin-GPR54 signaling in mouse NO-synthesizing neurons participates in the hypothalamic control of ovulation.J Neurosci. 2012 Jan 18;32(3):932-45. doi: 10.1523/JNEUROSCI.4765-11.2012. J Neurosci. 2012. PMID: 22262891 Free PMC article.
-
Leptin's effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in Kiss1 neurons.J Clin Invest. 2011 Jan;121(1):355-68. doi: 10.1172/JCI45106. Epub 2010 Dec 22. J Clin Invest. 2011. PMID: 21183787 Free PMC article.
-
Leptin signaling in GABA neurons, but not glutamate neurons, is required for reproductive function.J Neurosci. 2013 Nov 6;33(45):17874-83. doi: 10.1523/JNEUROSCI.2278-13.2013. J Neurosci. 2013. PMID: 24198376 Free PMC article.
-
[Kisspeptin and leptin in the regulation of fertility].Mol Biol (Mosk). 2015 Sep-Oct;49(5):707-15. doi: 10.7868/S0026898415050134. Mol Biol (Mosk). 2015. PMID: 26510589 Review. Russian.
-
The KiNG of reproduction: Kisspeptin/ nNOS interactions shaping hypothalamic GnRH release.Mol Cell Endocrinol. 2021 Jul 15;532:111302. doi: 10.1016/j.mce.2021.111302. Epub 2021 May 5. Mol Cell Endocrinol. 2021. PMID: 33964320 Review.
Cited by
-
Comparative Hypothalamic Transcriptome Analysis Reveals Crucial mRNAs, lncRNAs, and circRNAs Affecting Litter Size in Goats.Genes (Basel). 2023 Feb 9;14(2):444. doi: 10.3390/genes14020444. Genes (Basel). 2023. PMID: 36833370 Free PMC article.
-
The Hypothalamic Preoptic Area and Body Weight Control.Neuroendocrinology. 2018;106(2):187-194. doi: 10.1159/000479875. Epub 2017 Aug 10. Neuroendocrinology. 2018. PMID: 28772276 Free PMC article. Review.
-
Long-term consequences of the absence of leptin signaling in early life.Elife. 2019 Jan 29;8:e40970. doi: 10.7554/eLife.40970. Elife. 2019. PMID: 30694175 Free PMC article.
-
Emerging Roles of Anti-Müllerian Hormone in Hypothalamic-Pituitary Function.Neuroendocrinology. 2019;109(3):218-229. doi: 10.1159/000500689. Epub 2019 Jul 5. Neuroendocrinology. 2019. PMID: 31280262 Free PMC article. Review.
-
Placental Regulation of Energy Homeostasis During Human Pregnancy.Endocrinology. 2020 Jul 1;161(7):bqaa076. doi: 10.1210/endocr/bqaa076. Endocrinology. 2020. PMID: 32417921 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

