CD36-mediated hematoma absorption following intracerebral hemorrhage: negative regulation by TLR4 signaling
- PMID: 24808360
- PMCID: PMC4049082
- DOI: 10.4049/jimmunol.1400054
CD36-mediated hematoma absorption following intracerebral hemorrhage: negative regulation by TLR4 signaling
Abstract
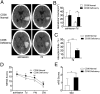
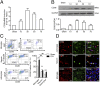
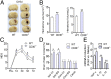
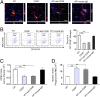
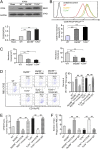
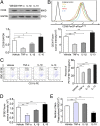
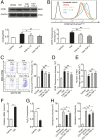
Promoting hematoma absorption is a novel therapeutic strategy for intracerebral hemorrhage (ICH); however, the mechanism of hematoma absorption is unclear. The present study explored the function and potential mechanism of CD36 in hematoma absorption using in vitro and in vivo ICH models. Hematoma absorption in CD36-deficient ICH patients was examined. Compared with patients with normal CD36 expression, CD36-deficient ICH patients had slower hematoma adsorption and aggravated neurologic deficits. CD36 expression in perihematomal tissues in wild-type mice following ICH was increased, whereas the hematoma absorption in CD36(-/-) mice was decreased. CD36(-/-) mice also showed aggravated neurologic deficits and increased TNF-α and IL-1β expression levels. The phagocytic capacity of CD36(-/-) microglia for RBCs was also decreased. Additionally, the CD36 expression in the perihematoma area after ICH in TLR4(-/-) and MyD88(-/-) mice was significantly increased, and hematoma absorption was significantly promoted, which was significantly inhibited by an anti-CD36 Ab. In vitro, TNF-α and IL-1β significantly inhibited the microglia expression of CD36 and reduced the microglia phagocytosis of RBCs. Finally, the TLR4 inhibitor TAK-242 upregulated CD36 expression in microglia, promoted hematoma absorption, increased catalase expression, and decreased the H2O2 content. These results suggested that CD36 mediated hematoma absorption after ICH, and TLR4 signaling inhibited CD36 expression to slow hematoma absorption. TLR4 inhibition could promote hematoma absorption and significantly improve neurologic deficits following ICH.
Copyright © 2014 by The American Association of Immunologists, Inc.
Figures
Similar articles
-
Naoxueshu oral liquid promotes hematoma absorption by targeting CD36 in M2 microglia via TLR4/MyD88/NF-κB signaling pathway in rats with intracerebral hemorrhage.J Ethnopharmacol. 2024 Jan 30;319(Pt 1):117116. doi: 10.1016/j.jep.2023.117116. Epub 2023 Sep 1. J Ethnopharmacol. 2024. PMID: 37659762
-
Cleaning up after ICH: the role of Nrf2 in modulating microglia function and hematoma clearance.J Neurochem. 2015 Apr;133(1):144-52. doi: 10.1111/jnc.12974. Epub 2014 Nov 24. J Neurochem. 2015. PMID: 25328080 Free PMC article.
-
Simvastatin accelerates hematoma resolution after intracerebral hemorrhage in a PPARγ-dependent manner.Neuropharmacology. 2018 Jan;128:244-254. doi: 10.1016/j.neuropharm.2017.10.021. Epub 2017 Oct 17. Neuropharmacology. 2018. PMID: 29054366
-
Research progress of endogenous hematoma absorption after intracerebral hemorrhage.Front Neurol. 2023 Mar 10;14:1115726. doi: 10.3389/fneur.2023.1115726. eCollection 2023. Front Neurol. 2023. PMID: 36970539 Free PMC article. Review.
-
The Critical Role of Erythrolysis and Microglia/Macrophages in Clot Resolution After Intracerebral Hemorrhage: A Review of the Mechanisms and Potential Therapeutic Targets.Cell Mol Neurobiol. 2023 Jan;43(1):59-67. doi: 10.1007/s10571-021-01175-3. Epub 2022 Jan 4. Cell Mol Neurobiol. 2023. PMID: 34981286 Review.
Cited by
-
An update on inflammation in the acute phase of intracerebral hemorrhage.Transl Stroke Res. 2015 Feb;6(1):4-8. doi: 10.1007/s12975-014-0384-4. Epub 2014 Dec 23. Transl Stroke Res. 2015. PMID: 25533878 Review.
-
Neuroprotective effect of Naochuxue prescription on intracerebral hemorrhage: inhibition of autophagy downregulating high mobility group box-1.J Tradit Chin Med. 2024 Oct;44(5):944-953. doi: 10.19852/j.cnki.jtcm.20240515.003. J Tradit Chin Med. 2024. PMID: 39380225 Free PMC article.
-
Recurrent Chronic Subdural Hematoma: Report of 13 Cases.Open Med (Wars). 2018 Oct 22;13:520-527. doi: 10.1515/med-2018-0076. eCollection 2018. Open Med (Wars). 2018. PMID: 30426091 Free PMC article.
-
Microglia Phenotype and Intracerebral Hemorrhage: A Balance of Yin and Yang.Front Cell Neurosci. 2021 Oct 13;15:765205. doi: 10.3389/fncel.2021.765205. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34720885 Free PMC article.
-
Prdx1 Reduces Intracerebral Hemorrhage-Induced Brain Injury via Targeting Inflammation- and Apoptosis-Related mRNA Stability.Front Neurosci. 2020 Mar 10;14:181. doi: 10.3389/fnins.2020.00181. eCollection 2020. Front Neurosci. 2020. PMID: 32210752 Free PMC article.
References
-
- Zhao X., Sun G., Zhang J., Strong R., Song W., Gonzales N., Grotta J. C., Aronowski J. 2007. Hematoma resolution as a target for intracerebral hemorrhage treatment: role for peroxisome proliferator-activated receptor γ in microglia/macrophages. Ann. Neurol. 61: 352–362 - PubMed
-
- Zhao X., Grotta J., Gonzales N., Aronowski J. 2009. Hematoma resolution as a therapeutic target: the role of microglia/macrophages. Stroke 40(3, Suppl. 1): S92–S94 - PubMed
-
- Nyquist P. 2010. Management of acute intracranial and intraventricular hemorrhage. Crit. Care Med. 38: 946–953 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

