Inflammasome activation causes dual recruitment of NLRC4 and NLRP3 to the same macromolecular complex
- PMID: 24803432
- PMCID: PMC4034195
- DOI: 10.1073/pnas.1402911111
Inflammasome activation causes dual recruitment of NLRC4 and NLRP3 to the same macromolecular complex
Abstract
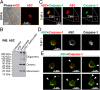
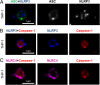
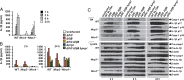
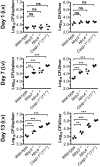
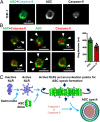
Pathogen recognition by nucleotide-binding oligomerization domain-like receptor (NLR) results in the formation of a macromolecular protein complex (inflammasome) that drives protective inflammatory responses in the host. It is thought that the number of inflammasome complexes forming in a cell is determined by the number of NLRs being activated, with each NLR initiating its own inflammasome assembly independent of one another; however, we show here that the important foodborne pathogen Salmonella enterica serovar Typhimurium (S. Typhimurium) simultaneously activates at least two NLRs, whereas only a single inflammasome complex is formed in a macrophage. Both nucleotide-binding domain and leucine-rich repeat caspase recruitment domain 4 and nucleotide-binding domain and leucine-rich repeat pyrin domain 3 are simultaneously present in the same inflammasome, where both NLRs are required to drive IL-1β processing within the Salmonella-infected cell and to regulate the bacterial burden in mice. Superresolution imaging of Salmonella-infected macrophages revealed a macromolecular complex with an outer ring of apoptosis-associated speck-like protein containing a caspase activation and recruitment domain and an inner ring of NLRs, with active caspase effectors containing the pro-IL-1β substrate localized internal to the ring structure. Our data reveal the spatial localization of different components of the inflammasome and how different members of the NLR family cooperate to drive robust IL-1β processing during Salmonella infection.
Keywords: ASC; bacteria; caspase-1; caspase-8; innate immunity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Salmonella infection induces recruitment of Caspase-8 to the inflammasome to modulate IL-1β production.J Immunol. 2013 Nov 15;191(10):5239-46. doi: 10.4049/jimmunol.1301581. Epub 2013 Oct 11. J Immunol. 2013. PMID: 24123685 Free PMC article.
-
The CARD plays a critical role in ASC foci formation and inflammasome signalling.Biochem J. 2013 Feb 1;449(3):613-21. doi: 10.1042/BJ20121198. Biochem J. 2013. PMID: 23110696 Free PMC article.
-
Caspase-1 autoproteolysis is differentially required for NLRP1b and NLRP3 inflammasome function.Proc Natl Acad Sci U S A. 2014 Dec 2;111(48):17254-9. doi: 10.1073/pnas.1415756111. Epub 2014 Nov 17. Proc Natl Acad Sci U S A. 2014. PMID: 25404286 Free PMC article.
-
Advances in Understanding Activation and Function of the NLRC4 Inflammasome.Int J Mol Sci. 2021 Jan 21;22(3):1048. doi: 10.3390/ijms22031048. Int J Mol Sci. 2021. PMID: 33494299 Free PMC article. Review.
-
Mechanisms of NOD-like receptor-associated inflammasome activation.Immunity. 2013 Sep 19;39(3):432-41. doi: 10.1016/j.immuni.2013.08.037. Immunity. 2013. PMID: 24054327 Free PMC article. Review.
Cited by
-
International Union of Basic and Clinical Pharmacology. XCVI. Pattern recognition receptors in health and disease.Pharmacol Rev. 2015;67(2):462-504. doi: 10.1124/pr.114.009928. Pharmacol Rev. 2015. PMID: 25829385 Free PMC article. Review.
-
ATP binding by NLRP7 is required for inflammasome activation in response to bacterial lipopeptides.Mol Immunol. 2015 Oct;67(2 Pt B):294-302. doi: 10.1016/j.molimm.2015.06.013. Epub 2015 Jul 2. Mol Immunol. 2015. PMID: 26143398 Free PMC article.
-
The inflammasome NLRP3 plays a dual role on mouse corpora cavernosa relaxation.Sci Rep. 2019 Nov 7;9(1):16224. doi: 10.1038/s41598-019-52831-0. Sci Rep. 2019. PMID: 31700106 Free PMC article.
-
Salmonella-Infected Aortic Aneurysm: Investigating Pathogenesis Using Salmonella Serotypes.Pol J Microbiol. 2019 Dec;68(4):439-447. doi: 10.33073/pjm-2019-043. Epub 2019 Oct 22. Pol J Microbiol. 2019. PMID: 31880888 Free PMC article.
-
Genetic ablation of diabetes-associated gene Ccdc92 reduces obesity and insulin resistance in mice.iScience. 2022 Dec 8;26(1):105769. doi: 10.1016/j.isci.2022.105769. eCollection 2023 Jan 20. iScience. 2022. PMID: 36594018 Free PMC article.
References
-
- McCoy AJ, Koizumi Y, Higa N, Suzuki T. Differential regulation of caspase-1 activation via NLRP3/NLRC4 inflammasomes mediated by aerolysin and type III secretion system during Aeromonas veronii infection. J Immunol. 2010;185(11):7077–7084. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

