Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function
- PMID: 24792912
- PMCID: PMC4513358
- DOI: 10.1016/j.immuni.2014.03.011
Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function
Abstract
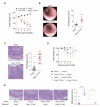
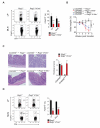
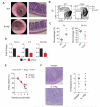
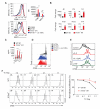
Intact interleukin-10 receptor (IL-10R) signaling on effector and T regulatory (Treg) cells are each independently required to maintain immune tolerance. Here we show that IL-10 sensing by innate immune cells, independent of its effects on T cells, was critical for regulating mucosal homeostasis. Following wild-type (WT) CD4(+) T cell transfer, Rag2(-/-)Il10rb(-/-) mice developed severe colitis in association with profound defects in generation and function of Treg cells. Moreover, loss of IL-10R signaling impaired the generation and function of anti-inflammatory intestinal and bone-marrow-derived macrophages and their ability to secrete IL-10. Importantly, transfer of WT but not Il10rb(-/-) anti-inflammatory macrophages ameliorated colitis induction by WT CD4(+) T cells in Rag2(-/-)Il10rb(-/-) mice. Similar alterations in the generation and function of anti-inflammatory macrophages were observed in IL-10R-deficient patients with very early onset inflammatory bowel disease. Collectively, our studies define innate immune IL-10R signaling as a key factor regulating mucosal immune homeostasis in mice and humans.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures

Comment in
-
IL-10 and macrophages orchestrate gut homeostasis.Immunity. 2014 May 15;40(5):637-9. doi: 10.1016/j.immuni.2014.04.015. Immunity. 2014. PMID: 24837099
Similar articles
-
Interleukin 1β Mediates Intestinal Inflammation in Mice and Patients With Interleukin 10 Receptor Deficiency.Gastroenterology. 2016 Dec;151(6):1100-1104. doi: 10.1053/j.gastro.2016.08.055. Epub 2016 Sep 28. Gastroenterology. 2016. PMID: 27693323 Free PMC article.
-
Macrophage-restricted interleukin-10 receptor deficiency, but not IL-10 deficiency, causes severe spontaneous colitis.Immunity. 2014 May 15;40(5):720-33. doi: 10.1016/j.immuni.2014.03.012. Epub 2014 May 1. Immunity. 2014. PMID: 24792913
-
Re-evaluation of IL-10 signaling reveals novel insights on the contribution of the intracellular domain of the IL-10R2 chain.PLoS One. 2017 Oct 10;12(10):e0186317. doi: 10.1371/journal.pone.0186317. eCollection 2017. PLoS One. 2017. PMID: 29016674 Free PMC article.
-
Vascular Endothelial Cells and Innate Immunity.Arterioscler Thromb Vasc Biol. 2020 Jun;40(6):e138-e152. doi: 10.1161/ATVBAHA.120.314330. Epub 2020 May 27. Arterioscler Thromb Vasc Biol. 2020. PMID: 32459541 Free PMC article. Review.
-
The molecular basis of IL-10 function: from receptor structure to the onset of signaling.Curr Top Microbiol Immunol. 2014;380:191-212. doi: 10.1007/978-3-662-43492-5_9. Curr Top Microbiol Immunol. 2014. PMID: 25004819 Free PMC article. Review.
Cited by
-
Intestinal Macrophages at the Crossroad between Diet, Inflammation, and Cancer.Int J Mol Sci. 2020 Jul 8;21(14):4825. doi: 10.3390/ijms21144825. Int J Mol Sci. 2020. PMID: 32650452 Free PMC article. Review.
-
Congenital diarrhoeal disorders: advances in this evolving web of inherited enteropathies.Nat Rev Gastroenterol Hepatol. 2015 May;12(5):293-302. doi: 10.1038/nrgastro.2015.44. Epub 2015 Mar 17. Nat Rev Gastroenterol Hepatol. 2015. PMID: 25782092 Free PMC article. Review.
-
Interleukin 1β Mediates Intestinal Inflammation in Mice and Patients With Interleukin 10 Receptor Deficiency.Gastroenterology. 2016 Dec;151(6):1100-1104. doi: 10.1053/j.gastro.2016.08.055. Epub 2016 Sep 28. Gastroenterology. 2016. PMID: 27693323 Free PMC article.
-
Macrophage MMP10 Regulates TLR7-Mediated Tolerance.Front Immunol. 2018 Dec 4;9:2817. doi: 10.3389/fimmu.2018.02817. eCollection 2018. Front Immunol. 2018. PMID: 30564235 Free PMC article.
-
CCR2 promotes monocyte recruitment and intestinal inflammation in mice lacking the interleukin-10 receptor.Sci Rep. 2022 Jan 10;12(1):452. doi: 10.1038/s41598-021-04098-7. Sci Rep. 2022. PMID: 35013585 Free PMC article.
References
-
- Bain CC, Scott CL, Uronen-Hansson H, Gudjonsson S, Jansson O, Grip O, Guilliams M, Malissen B, Agace WW, Mowat AM. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal immunology. 2013;6:498–510. - PMC - PubMed
-
- Begue B, Verdier J, Rieux-Laucat F, Goulet O, Morali A, Canioni D, Hugot JP, Daussy C, Verkarre V, Pigneur B, et al. Defective IL10 signaling defining a subgroup of patients with inflammatory bowel disease. The American journal of gastroenterology. 2011;106:1544–1555. - PubMed
-
- Bhattacharyya S, Sen P, Wallet M, Long B, Baldwin AS, Jr., Tisch R. Immunoregulation of dendritic cells by IL-10 is mediated through suppression of the PI3K/Akt pathway and of IkappaB kinase activity. Blood. 2004;104:1100–1109. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

