SUMOylation of Grb2 enhances the ERK activity by increasing its binding with Sos1
- PMID: 24775912
- PMCID: PMC4021559
- DOI: 10.1186/1476-4598-13-95
SUMOylation of Grb2 enhances the ERK activity by increasing its binding with Sos1
Abstract
Background: Grb2 (Growth factor receptor-bound protein 2) is a key adaptor protein in maintaining the ERK activity via linking Sos1 (Son of sevenless homolog 1) or other proteins to activated RTKs, such as EGFR. Currently, little knowledge is available concerning the post-translational modification (PTM) of Grb2 except for its phosphorylation. Since emerging evidences have highlighted the importance of SUMOylation (Small ubiquitin-related modifier), a reversible PTM, in modulating protein functions, we wondered if Grb2 could be SUMOylated and thereby influences its functions especially involved in the Ras/MEK/ERK pathway.
Methods: SUMOylation of Grb2 was analyzed with the in vivo SUMOylation assay using the Ni2+-NTA affinity pulldown and the in vitro E.coli-based SUMOylation assay. To test the ERK activity and cell transformation, the murine fibroblast cell line NIH/3T3 and the murine colon cancer cell line CMT-93 were used for the experiments including Grb2 knockdown, ectopic re-expression, cell transformation and migration. Immunoprecipitation (IP) was employed for seeking proteins that interact with SUMO modified Grb2. Xenograft tumor model in mice was conducted to verify that Grb2 SUMOylation regulated tumorigenesis in vivo.
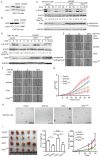
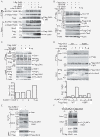
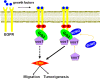
Results: Grb2 can be SUMOylated by SUMO1 at lysine 56 (K56), which is located in the linker region between the N-terminal SH3 domain and the SH2 domain. Knockdown of Grb2 reduced the ERK activity and suppressed cell motility and tumorigenesis in vitro and in vivo, which were all rescued by stable ectopic re-expression of wild-type Grb2 but not the mutant Grb2K56R. Furthermore, Grb2 SUMOylation at K56 increased the formation of Grb2-Sos1 complex, which sequentially leads to the activation of Ras/MEK/MAPK pathway.
Conclusions: Our results provide evidences that Grb2 is SUMOylated in vivo and this modification enhances ERK activities via increasing the formation of Grb2-Sos1 complex, and may consequently promote cell motility, transformation and tumorigenesis.
Figures



Similar articles
-
The ability of Sos1 to oligomerize the adaptor protein LAT is separable from its guanine nucleotide exchange activity in vivo.Sci Signal. 2013 Nov 12;6(301):ra99. doi: 10.1126/scisignal.2004494. Sci Signal. 2013. PMID: 24222714 Free PMC article.
-
Small Molecule-Mediated Activation of RAS Elicits Biphasic Modulation of Phospho-ERK Levels that Are Regulated through Negative Feedback on SOS1.Mol Cancer Ther. 2018 May;17(5):1051-1060. doi: 10.1158/1535-7163.MCT-17-0666. Epub 2018 Feb 13. Mol Cancer Ther. 2018. PMID: 29440291
-
Targeted Covalent Inhibition of Grb2-Sos1 Interaction through Proximity-Induced Conjugation in Breast Cancer Cells.Mol Pharm. 2017 May 1;14(5):1548-1557. doi: 10.1021/acs.molpharmaceut.6b00952. Epub 2017 Jan 17. Mol Pharm. 2017. PMID: 28060514
-
Tnk1/Kos1: a novel tumor suppressor.Trans Am Clin Climatol Assoc. 2010;121:281-92; discussion 292-3. Trans Am Clin Climatol Assoc. 2010. PMID: 20697568 Free PMC article. Review.
-
The Configuration of GRB2 in Protein Interaction and Signal Transduction.Biomolecules. 2024 Feb 22;14(3):259. doi: 10.3390/biom14030259. Biomolecules. 2024. PMID: 38540680 Free PMC article. Review.
Cited by
-
SUMOylation of Csk Negatively Modulates its Tumor Suppressor Function.Neoplasia. 2019 Jul;21(7):676-688. doi: 10.1016/j.neo.2019.04.010. Epub 2019 May 21. Neoplasia. 2019. PMID: 31125786 Free PMC article.
-
Shp2 SUMOylation promotes ERK activation and hepatocellular carcinoma development.Oncotarget. 2015 Apr 20;6(11):9355-69. doi: 10.18632/oncotarget.3323. Oncotarget. 2015. PMID: 25823821 Free PMC article.
-
SUMOylation and Viral Infections of the Brain.Pathogens. 2022 Jul 21;11(7):818. doi: 10.3390/pathogens11070818. Pathogens. 2022. PMID: 35890062 Free PMC article. Review.
-
RNF173 suppresses RAF/MEK/ERK signaling to regulate invasion and metastasis via GRB2 ubiquitination in Hepatocellular Carcinoma.Cell Commun Signal. 2023 Aug 25;21(1):224. doi: 10.1186/s12964-023-01241-x. Cell Commun Signal. 2023. PMID: 37626338 Free PMC article.
-
Atypical ubiquitin E3 ligase complex Skp1-Pam-Fbxo45 controls the core epithelial-to-mesenchymal transition-inducing transcription factors.Oncotarget. 2015 Jan 20;6(2):979-94. doi: 10.18632/oncotarget.2825. Oncotarget. 2015. PMID: 25460509 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

