Comparison of frequencies and prognostic effect of molecular subtypes between young and elderly breast cancer patients
- PMID: 24767310
- PMCID: PMC5528523
- DOI: 10.1016/j.molonc.2014.03.022
Comparison of frequencies and prognostic effect of molecular subtypes between young and elderly breast cancer patients
Abstract
Purpose: To compare the distribution and prognostic effect of the breast cancer molecular subtypes in young and elderly breast cancer patients.
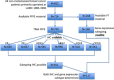
Patients and methods: Our study population (n = 822) consisted of all early breast cancer patients primarily treated with surgery in our center between 1985 and 1996. A total of 142/822 fresh frozen tissues were available with good quality RNA and analyzed by gene expression microarray. Gene expression molecular subtypes were determined by correlation to the expression centroids of 534 "intrinsic" genes. Sections of a tissue micro array containing formalin-fixed paraffin-embedded tumor tissue of 714/822 patients were immunohistochemically (IHC) stained for Ki67, EGFR, CK5/6. Tumor expression of ER, PR, HER2 was previously determined. IHC molecular subtypes were defined based on expression of these markers: Luminal A: ER+ and/or PR+, HER2- and Ki67-; Luminal B: ER+ and/or PR+ and ki67+; ERBB2: ER-, PR- and HER2+; Basal-like: ER-, PR-, HER2- and EGFR+ and/or CK5/6+; Unclassified: ER-, PR-, HER2-, EGFR- and CK5/6-. IHC molecular subtypes were validated against gene expression defined molecular subtypes. Assessment of distribution and prognostic effect of molecular subtypes was stratified to age (<65 versus ≥65 years).
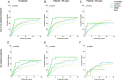
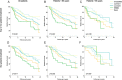
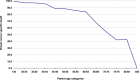
Results: Validation of molecular subtypes determined by IHC against gene expression revealed a substantial agreement in classification (Cohen's kappa coefficient 0.75). A statistically significant association (p = 0.02) was found between molecular subtypes and age, where Luminal tumors were more often found in elderly patients, while ERBB2, basal-like and unclassified subtypes were more often found in young patients. Molecular subtypes showed a prognostic association with outcome in young patients concerning relapse-free period (RFP) (p = 0.01) and relative survival (RS) (p < 0.001). No statistically significant prognostic effect was found for molecular subtypes in elderly patients (RFP p = 0.5; RS p = 0.1). Additional analyses showed that no molecular subtypes showed a statistically significant difference in outcome for elderly compare to young patients.
Conclusion: We have shown that molecular subtypes have a different distribution and prognostic effect in elderly compared to young breast cancer patients, emphasizing the fact that biomarkers may have different distributions and prognostic effects and therefore different implications in elderly compared to their younger counterparts. Our results support the premise that breast cancer clinical behavior is significantly affected by patient age. We suggest that competing risks of death in elderly patients, ER-driven differences and micro-environmental changes in biology are underlying these age-dependent variations in patient prognosis.
Keywords: Breast cancer; Elderly; Molecular subtypes; Prognostication.
Copyright © 2014 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures

Similar articles
-
The clinical prognostic value of molecular intrinsic tumor subtypes in older breast cancer patients: A FOCUS study analysis.Mol Oncol. 2016 Apr;10(4):594-600. doi: 10.1016/j.molonc.2015.11.002. Epub 2015 Nov 24. Mol Oncol. 2016. PMID: 26706834 Free PMC article.
-
PAM50 breast cancer subtyping by RT-qPCR and concordance with standard clinical molecular markers.BMC Med Genomics. 2012 Oct 4;5:44. doi: 10.1186/1755-8794-5-44. BMC Med Genomics. 2012. PMID: 23035882 Free PMC article.
-
Breast cancer-specific survival by clinical subtype after 7 years follow-up of young and elderly women in a nationwide cohort.Int J Cancer. 2019 Mar 15;144(6):1251-1261. doi: 10.1002/ijc.31950. Epub 2018 Dec 3. Int J Cancer. 2019. PMID: 30367449
-
Biology of primary breast cancer in older women beyond routine biomarkers.Breast Cancer. 2021 Sep;28(5):991-1001. doi: 10.1007/s12282-021-01266-5. Epub 2021 Jun 24. Breast Cancer. 2021. PMID: 34165702 Free PMC article. Review.
-
Breast Cancer Molecular Stratification: From Intrinsic Subtypes to Integrative Clusters.Am J Pathol. 2017 Oct;187(10):2152-2162. doi: 10.1016/j.ajpath.2017.04.022. Epub 2017 Jul 19. Am J Pathol. 2017. PMID: 28733194 Review.
Cited by
-
Clinical Characteristics and Survival Outcomes of Infiltrating Lobular Carcinoma: A Retrospective Study of 365 Cases in China.Cancer Manag Res. 2022 Feb 16;14:647-658. doi: 10.2147/CMAR.S346319. eCollection 2022. Cancer Manag Res. 2022. PMID: 35210861 Free PMC article.
-
Nipple-sparing mastectomy in young versus elderly patients.Rev Bras Ginecol Obstet. 2024 Oct 23;46:e-rbgo90. doi: 10.61622/rbgo/2024rbgo90. eCollection 2024. Rev Bras Ginecol Obstet. 2024. PMID: 39530073 Free PMC article.
-
Cardiac toxicity of trastuzumab in elderly patients with breast cancer.J Geriatr Cardiol. 2016 May;13(4):355-63. doi: 10.11909/j.issn.1671-5411.2016.04.003. J Geriatr Cardiol. 2016. PMID: 27403145 Free PMC article. Review.
-
Impact of age on indication for chemotherapy in early breast cancer patients: results from 104 German institutions from 2008 to 2017.Arch Gynecol Obstet. 2023 Jul;308(1):219-229. doi: 10.1007/s00404-022-06902-9. Epub 2023 Jan 5. Arch Gynecol Obstet. 2023. PMID: 36604331 Free PMC article.
-
Clinically relevant gene signatures provide independent prognostic information in older breast cancer patients.Breast Cancer Res. 2024 Mar 7;26(1):38. doi: 10.1186/s13058-024-01797-7. Breast Cancer Res. 2024. PMID: 38454481 Free PMC article.
References
-
- Bastiaannet, E. , Liefers, G.J. , de Craen, A.J. , Kuppen, P.J. , van de Water, W. , Portielje, J.E. , van der Geest, L.G. , Janssen-Heijnen, M.L. , Dekkers, O.M. , van de Velde, C.J. , Westendorp, R.G. , 2010. Breast cancer in elderly compared to younger patients in The Netherlands: stage at diagnosis, treatment and survival in 127,805 unselected patients. Breast Cancer Res. Treat.. 124, 801–807. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

