Nuclear envelope lamin-A couples actin dynamics with immunological synapse architecture and T cell activation
- PMID: 24757177
- PMCID: PMC4337980
- DOI: 10.1126/scisignal.2004872
Nuclear envelope lamin-A couples actin dynamics with immunological synapse architecture and T cell activation
Abstract
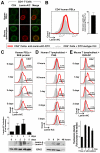
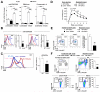
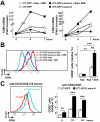
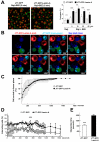
In many cell types, nuclear A-type lamins regulate multiple cellular functions, including higher-order genome organization, DNA replication and repair, gene transcription, and signal transduction; however, their role in specialized immune cells remains largely unexplored. We showed that the abundance of A-type lamins was almost negligible in resting naïve T lymphocytes, but was increased upon activation of the T cell receptor (TCR). The increase in lamin-A was an early event that accelerated formation of the immunological synapse between T cells and antigen-presenting cells. Polymerization of F-actin in T cells is a critical step for immunological synapse formation, and lamin-A interacted with the linker of nucleoskeleton and cytoskeleton (LINC) complex to promote F-actin polymerization. We also showed that lamin-A expression accelerated TCR clustering and led to enhanced downstream signaling, including extracellular signal-regulated kinase 1/2 (ERK1/2) signaling, as well as increased target gene expression. Pharmacological inhibition of the ERK pathway reduced lamin-A-dependent T cell activation. Moreover, mice lacking lamin-A in immune cells exhibited impaired T cell responses in vivo. These findings underscore the importance of A-type lamins for TCR activation and identify lamin-A as a previously unappreciated regulator of the immune response.
Figures
Comment in
-
T cells: Lamin A links synapse to nucleus.Nat Rev Immunol. 2014 Jun;14(6):356. doi: 10.1038/nri3692. Epub 2014 May 16. Nat Rev Immunol. 2014. PMID: 24830345 No abstract available.
Similar articles
-
T cells: Lamin A links synapse to nucleus.Nat Rev Immunol. 2014 Jun;14(6):356. doi: 10.1038/nri3692. Epub 2014 May 16. Nat Rev Immunol. 2014. PMID: 24830345 No abstract available.
-
Multiple actin networks coordinate mechanotransduction at the immunological synapse.J Cell Biol. 2020 Feb 3;219(2):e201911058. doi: 10.1083/jcb.201911058. J Cell Biol. 2020. PMID: 31977034 Free PMC article.
-
NF-κB inducing kinase (NIK) is an essential post-transcriptional regulator of T-cell activation affecting F-actin dynamics and TCR signaling.J Autoimmun. 2018 Nov;94:110-121. doi: 10.1016/j.jaut.2018.07.017. Epub 2018 Jul 29. J Autoimmun. 2018. PMID: 30061013
-
Nuclear envelope lamin-A as a coordinator of T cell activation.Nucleus. 2014 Sep-Oct;5(5):396-401. doi: 10.4161/nucl.36361. Nucleus. 2014. PMID: 25482193 Free PMC article. Review.
-
New insights into the T cell synapse from single molecule techniques.Nat Rev Immunol. 2011 Sep 9;11(10):672-84. doi: 10.1038/nri3066. Nat Rev Immunol. 2011. PMID: 21904389 Free PMC article. Review.
Cited by
-
Tumor-specific T cells support chemokine-driven spatial organization of intratumoral immune microaggregates needed for long survival.J Immunother Cancer. 2022 Feb;10(2):e004346. doi: 10.1136/jitc-2021-004346. J Immunother Cancer. 2022. PMID: 35217577 Free PMC article.
-
The Nuclear Envelope as a Regulator of Immune Cell Function.Front Immunol. 2022 Jun 10;13:840069. doi: 10.3389/fimmu.2022.840069. eCollection 2022. Front Immunol. 2022. PMID: 35757775 Free PMC article. Review.
-
Nanotube patterning reduces macrophage inflammatory response via nuclear mechanotransduction.J Nanobiotechnology. 2023 Jul 19;21(1):229. doi: 10.1186/s12951-023-01912-4. J Nanobiotechnology. 2023. PMID: 37468894 Free PMC article.
-
Integrin α4β1 controls G9a activity that regulates epigenetic changes and nuclear properties required for lymphocyte migration.Nucleic Acids Res. 2016 Apr 20;44(7):3031-44. doi: 10.1093/nar/gkv1348. Epub 2015 Dec 10. Nucleic Acids Res. 2016. PMID: 26657637 Free PMC article.
-
Mechanoimmunology: Are inflammatory epigenetic states of macrophages tuned by biophysical factors?APL Bioeng. 2022 Aug 29;6(3):031502. doi: 10.1063/5.0087699. eCollection 2022 Sep. APL Bioeng. 2022. PMID: 36051106 Free PMC article. Review.
References
-
- Broers JL, Ramaekers FC, Bonne G, Yaou RB, Hutchison CJ. Nuclear lamins: laminopathies and their role in premature ageing. Physiol Rev. 2006;86:967–1008. - PubMed
-
- Houben F, Willems CH, Declercq IL, Hochstenbach K, Kamps MA, Snoeckx LH, Ramaekers FC, Broers JL. Disturbed nuclear orientation and cellular migration in A-type lamin deficient cells. Biochim Biophys Acta. 2009;1793:312–324. - PubMed
-
- Burke B, Stewart CL. The nuclear lamins: flexibility in function. Nat Rev Mol Cell Biol. 2012;14:13–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

