Role of heparan sulfate proteoglycans in optic disc and stalk morphogenesis
- PMID: 24753163
- PMCID: PMC4177358
- DOI: 10.1002/dvdy.24142
Role of heparan sulfate proteoglycans in optic disc and stalk morphogenesis
Abstract
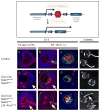
Background: Heparan sulfate proteoglycans (HSPG) are important for embryonic development by means of the regulation of gradient formation and signaling of multiple growth factors and morphogens. Previous studies have shown that Bmp/Shh/Fgf signaling are required for the regionalization of the optic vesicle (OV) and for the closure of the optic fissure (OF), the disturbance of which underlie ocular anomalies such as microphthalmia, coloboma, and optic nerve hypoplasia.
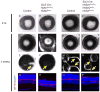
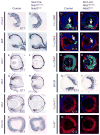
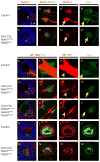
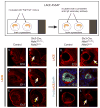
Results: To study HSPG-dependent coordination of these signaling pathways during mammalian visual system development, we have generated a series of OV-specific mutations in the heparan sulfate (HS) N-sulfotransferase genes (Ndst1 and Ndst2) and HS O-sulfotransferase genes (Hs2st, Hs6st1, and Hs6st2) in mice. Of interest, the resulting HS undersulfation still allowed for normal retinal neurogenesis and optic fissure closure, but led to defective optic disc and stalk development. The adult mutant animals further developed optic nerve aplasia/hypoplasia and displayed retinal degeneration. We observed that MAPK/ERK signaling was down-regulated in Ndst mutants, and consistent with this, HS-related optic nerve morphogenesis defects in mutant mice could partially be rescued by constitutive Kras activation.
Conclusions: These results suggest that HSPGs, depending on their HS sulfation pattern, regulate multiple signaling pathways in optic disc and stalk morphogenesis.
Keywords: Fgf; HSPG; Ndst; optic disc; optic stalk; sulfotransferase.
Copyright © 2014 Wiley Periodicals, Inc.
Figures
Similar articles
-
Heparan sulphation patterns generated by specific heparan sulfotransferase enzymes direct distinct aspects of retinal axon guidance at the optic chiasm.J Neurosci. 2006 Jun 28;26(26):6911-23. doi: 10.1523/JNEUROSCI.0505-06.2006. J Neurosci. 2006. PMID: 16807321 Free PMC article.
-
Enzymatically active N-deacetylase/N-sulfotransferase-2 is present in liver but does not contribute to heparan sulfate N-sulfation.J Biol Chem. 2006 Nov 24;281(47):35727-34. doi: 10.1074/jbc.M604113200. Epub 2006 Sep 19. J Biol Chem. 2006. PMID: 16984905
-
Lacrimal gland development and Fgf10-Fgfr2b signaling are controlled by 2-O- and 6-O-sulfated heparan sulfate.J Biol Chem. 2011 Apr 22;286(16):14435-44. doi: 10.1074/jbc.M111.225003. Epub 2011 Feb 28. J Biol Chem. 2011. PMID: 21357686 Free PMC article.
-
[Exploration of the molecular mechanism of ocular development and the creation of animal models for ocular diseases].Nippon Ganka Gakkai Zasshi. 2010 Mar;114(3):280-96; discussion 297. Nippon Ganka Gakkai Zasshi. 2010. PMID: 20387539 Review. Japanese.
-
Genes and pathways in optic fissure closure.Semin Cell Dev Biol. 2019 Jul;91:55-65. doi: 10.1016/j.semcdb.2017.10.010. Epub 2017 Dec 6. Semin Cell Dev Biol. 2019. PMID: 29198497 Review.
Cited by
-
Multiple roles of epithelial heparan sulfate in stomach morphogenesis.J Cell Sci. 2018 May 29;131(10):jcs210781. doi: 10.1242/jcs.210781. J Cell Sci. 2018. PMID: 29700203 Free PMC article.
-
The role of heparan sulphate in development: the ectodermal story.Int J Exp Pathol. 2016 Jun;97(3):213-29. doi: 10.1111/iep.12180. Epub 2016 Jul 6. Int J Exp Pathol. 2016. PMID: 27385054 Free PMC article. Review.
-
MicroRNA-96: A therapeutic and diagnostic tumor marker.Iran J Basic Med Sci. 2022 Jan;25(1):3-13. doi: 10.22038/IJBMS.2021.59604.13226. Iran J Basic Med Sci. 2022. PMID: 35656454 Free PMC article. Review.
-
Chondroitin sulfate enhances the barrier function of basement membrane assembled by heparan sulfate.Development. 2022 Jun 15;149(12):dev200569. doi: 10.1242/dev.200569. Epub 2022 Jun 16. Development. 2022. PMID: 35608020 Free PMC article.
-
The Fibroblast Growth Factor signaling pathway.Wiley Interdiscip Rev Dev Biol. 2015 May-Jun;4(3):215-66. doi: 10.1002/wdev.176. Epub 2015 Mar 13. Wiley Interdiscip Rev Dev Biol. 2015. PMID: 25772309 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

