Natural killer cell and T-cell subset distributions and activation influence susceptibility to perinatal HIV-1 infection
- PMID: 24752082
- PMCID: PMC4365995
- DOI: 10.1097/QAD.0000000000000263
Natural killer cell and T-cell subset distributions and activation influence susceptibility to perinatal HIV-1 infection
Abstract
Objective: To determine neonatal immunologic factors that correlate with mother-to-child-transmission of HIV-1.
Design: This case-control study compared cord blood natural killer (NK) and T-cell populations of HIV-1 exposed infants who subsequently acquired infection by 1 month (cases) to those who remained uninfected by 1 year of life (controls). Control specimens were selected by proportional match on maternal viral load.
Methods: Cryopreserved cord blood mononuclear cells (CBMCs) were thawed and stained for multiparameter flow cytometry to detect NK and T-cell subsets and activation status. CBMCs were also used in a viral suppression assay to evaluate NK cell inhibition of HIV-1 replication in autologous CD4 T cells.
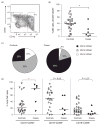
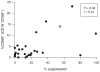
Results: Cord blood from cases contained a skewed NK cell repertoire characterized by an increased proportion of CD16CD56 NK cells. In addition, cases displayed less-activated CD16CD56 NK cells and CD8 T cells, based on HLA-DRCD38 costaining. NK cell suppression of HIV-1 replication ex vivo correlated with the proportion of acutely activated CD68CD16CD56 NK cells. Finally, we detected a higher proportion of CD27CD45RA effector memory CD4 and CD8 T cells in cord blood from cases compared with controls.
Conclusion: When controlled for maternal viral load, cord blood from infants who acquired HIV-1 had a higher proportion of CD16CD56 NK cells, lower NK cell activation and higher levels of mature T cells (potential HIV-1 targets) than control infants who remained uninfected. Our data provide evidence that infant HIV-1 acquisition may be influenced by both innate and adaptive immune cell phenotypes and activation status.
Conflict of interest statement
The authors declare there are no conflicts of interest.
Figures


Similar articles
-
Higher CCR5 density on CD4 + T-cells in mothers and infants is associated with increased risk of in-utero HIV-1 transmission.AIDS. 2024 Jun 1;38(7):945-954. doi: 10.1097/QAD.0000000000003857. Epub 2024 Feb 14. AIDS. 2024. PMID: 38329228 Free PMC article.
-
Potential Role of Regulatory T Cells in Mother-to-Child Transmission of HIV.Curr HIV Res. 2018;16(6):396-403. doi: 10.2174/1570162X17666190213094624. Curr HIV Res. 2018. PMID: 30760190 Free PMC article.
-
Increased Natural Killer Cell Activation in HIV-Infected Immunologic Non-Responders Correlates with CD4+ T Cell Recovery after Antiretroviral Therapy and Viral Suppression.PLoS One. 2017 Jan 11;12(1):e0167640. doi: 10.1371/journal.pone.0167640. eCollection 2017. PLoS One. 2017. PMID: 28076376 Free PMC article. Clinical Trial.
-
Evidence for the innate immune response as a correlate of protection in human immunodeficiency virus (HIV)-1 highly exposed seronegative subjects (HESN).Clin Exp Immunol. 2011 May;164(2):158-69. doi: 10.1111/j.1365-2249.2011.04379.x. Epub 2011 Mar 17. Clin Exp Immunol. 2011. PMID: 21413945 Free PMC article. Review.
-
Role of Early Life Cytotoxic T Lymphocyte and Natural Killer Cell Immunity in Paediatric HIV Cure/Remission in the Anti-Retroviral Therapy Era.Front Immunol. 2022 May 11;13:886562. doi: 10.3389/fimmu.2022.886562. eCollection 2022. Front Immunol. 2022. PMID: 35634290 Free PMC article. Review.
Cited by
-
Ontogeny of CD4+ T Lymphocytes With Phenotypic Susceptibility to HIV-1 During Exclusive and Nonexclusive Breastfeeding in HIV-1-Exposed Ugandan Infants.J Infect Dis. 2017 Feb 1;215(3):368-377. doi: 10.1093/infdis/jiw553. J Infect Dis. 2017. PMID: 27932619 Free PMC article.
-
Non-linear multidimensional flow cytometry analyses delineate NK cell phenotypes in normal and HIV-infected chimpanzees.Int Immunol. 2019 Mar 5;31(3):175-180. doi: 10.1093/intimm/dxy076. Int Immunol. 2019. PMID: 30418531 Free PMC article.
-
Defective Monocyte Enzymatic Function and an Inhibitory Immune Phenotype in Human Immunodeficiency Virus-Exposed Uninfected African Infants in the Era of Antiretroviral Therapy.J Infect Dis. 2022 Sep 28;226(7):1243-1255. doi: 10.1093/infdis/jiac133. J Infect Dis. 2022. PMID: 35403683 Free PMC article.
-
Recent Advancements in Antifibrotic Therapies for Regression of Liver Fibrosis.Cells. 2022 Apr 29;11(9):1500. doi: 10.3390/cells11091500. Cells. 2022. PMID: 35563807 Free PMC article. Review.
-
Natural killer cells in HIV-1 infection and therapy.AIDS. 2017 Nov 13;31(17):2317-2330. doi: 10.1097/QAD.0000000000001645. AIDS. 2017. PMID: 28926399 Free PMC article. Review.
References
-
- Dosekun O, Fox J. An overview of the relative risks of different sexual behaviours on HIV transmission. Curr Opin HIV AIDS. 2010;5:291–297. - PubMed
-
- Van Der Pol B, Kwok C, Pierre-Louis B, Rinaldi A, Salata RA, Chen PL, et al. Trichomonas vaginalis infection and human immunodeficiency virus acquisition in African women. J Infect Dis. 2008;197:548–554. - PubMed
-
- Embree JE, Njenga S, Datta P, Nagelkerke NJ, Ndinya-Achola JO, Mohammed Z, et al. Risk factors for postnatal mother-child transmission of HIV-1. AIDS. 2000;14:2535–2541. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

